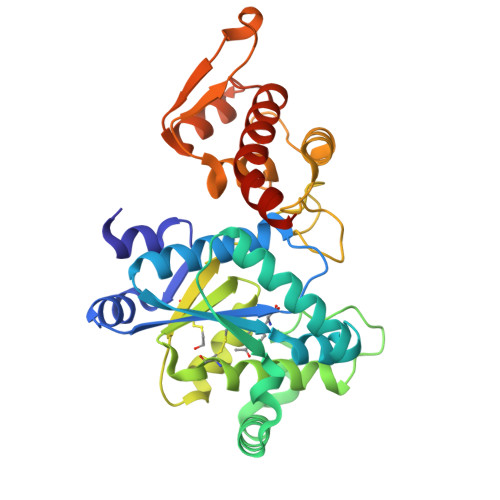
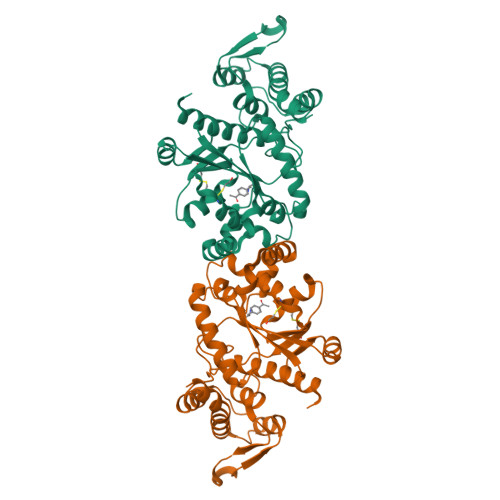
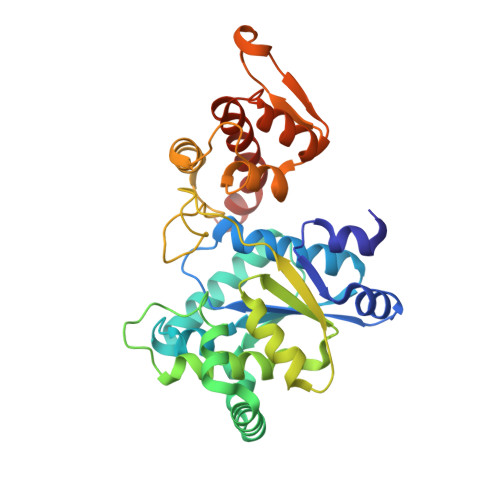
Structural characterization of a p-acetylphenylalanyl aminoacyl-tRNA synthetase.
Turner, J.M., Graziano, J., Spraggon, G., Schultz, P.G.(2005) J Am Chem Soc 127: 14976-14977
- PubMed: 16248607
- DOI: https://doi.org/10.1021/ja0549042
- Primary Citation of Related Structures:
1ZH6 - PubMed Abstract:
It has been recently shown that orthogonal tRNA/aminoacyl-tRNA synthetase pairs can be evolved to allow genetic incorporation of unnatural amino acids into proteins in both prokaryotes and eukaryotes. Here we describe the crystal structure of an evolved aminoacyl-tRNA synthetase that charges the unnatural amino acid p-acetylphenylalanine. Molecular recognition is due to altered hydrogen bonding and packing interactions with bound substrate that result from changes in both side-chain and backbone conformation.
Organizational Affiliation:
Department of Chemistry, The Scripps Research Institute, La Jolla, California 92037, USA.