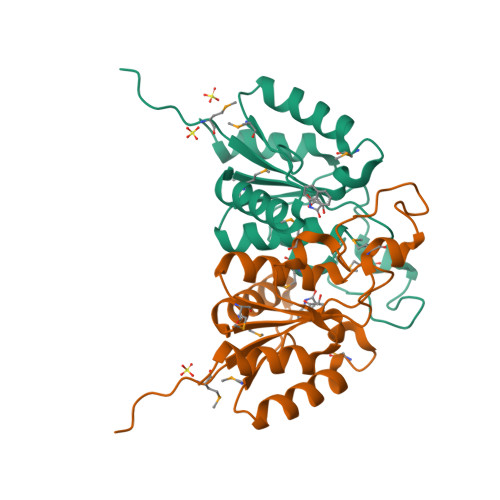
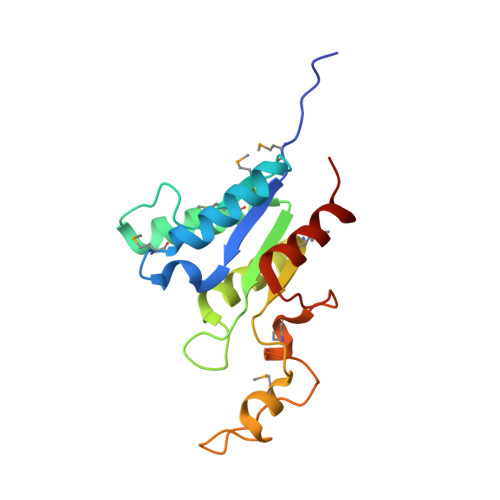
Using fragment cocktail crystallography to assist inhibitor design of Trypanosoma brucei nucleoside 2-deoxyribosyltransferase.
Bosch, J., Robien, M.A., Mehlin, C., Boni, E., Riechers, A., Buckner, F.S., Van Voorhis, W.C., Myler, P.J., Worthey, E.A., DeTitta, G., Luft, J.R., Lauricella, A., Gulde, S., Anderson, L.A., Kalyuzhniy, O., Neely, H.M., Ross, J., Earnest, T.N., Soltis, M., Schoenfeld, L., Zucker, F., Merritt, E.A., Fan, E., Verlinde, C.L., Hol, W.G.J.(2006) J Med Chem 49: 5939-5946
- PubMed: 17004709
- DOI: https://doi.org/10.1021/jm060429m
- Primary Citation of Related Structures:
2A0K, 2F2T, 2F62, 2F64, 2F67 - PubMed Abstract:
The 1.8 A resolution de novo structure of nucleoside 2-deoxyribosyltransferase (EC 2.4.2.6) from Trypanosoma brucei (TbNDRT) has been determined by SADa phasing in an unliganded state and several ligand-bound states. This enzyme is important in the salvage pathway of nucleoside recycling. To identify novel lead compounds, we exploited "fragment cocktail soaks". Out of 304 compounds tried in 31 cocktails, four compounds could be identified crystallographically in the active site. In addition, we demonstrated that very short soaks of approximately 10 s are sufficient even for rather hydrophobic ligands to bind in the active site groove, which is promising for the application of similar soaking experiments to less robust crystals of other proteins.
Organizational Affiliation:
Department of Biochemistry, Division of Infectious Disease, and Howard Hughes Medical Institute, University of Washington, Seattle, Washington 98195, USA.