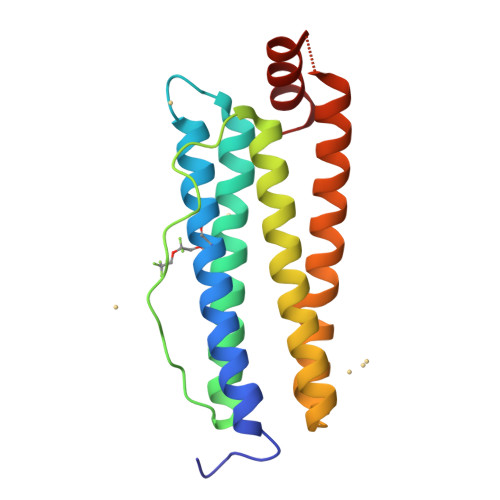
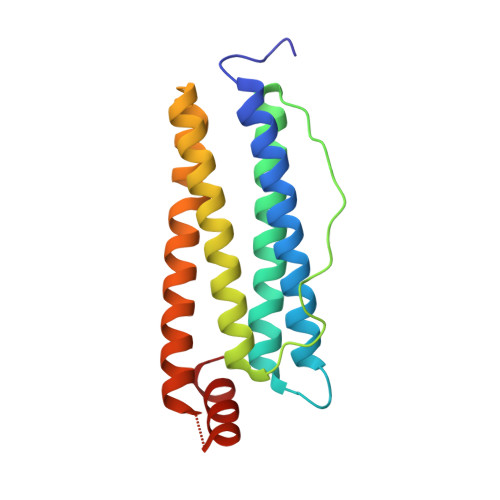
Photoactive analogues of the haloether anesthetics provide high-resolution features from low-affinity interactions.
Xi, J., Liu, R., Rossi, M.J., Yang, J., Loll, P.J., Dailey, W.P., Eckenhoff, R.G.(2006) ACS Chem Biol 1: 377-384
- PubMed: 17163775
- DOI: https://doi.org/10.1021/cb600207d
- Primary Citation of Related Structures:
2GYD - PubMed Abstract:
The difficulty in obtaining binding target and site information for low-affinity drugs, like the inhaled anesthetics, has limited identification of their molecular effectors. Because such information can be provided by photoactive analogues, we designed, synthesized, and characterized a novel diazirnyl haloether that closely mimics isoflurane, the most widely used clinical general anesthetic. This compound, H-diaziflurane, is a nontoxic, potent anesthetic that potentiates GABA-gated ion channels in primary cultures of hippocampal neurons. Calorimetric and structural characterizations show that H-diaziflurane binds a model anesthetic host protein with similar energetics as isoflurane and forms photoadducts with residues lining the isoflurane binding site. H-diaziflurane will be immediately useful for identifying targets and sites important for the molecular pharmacology of the inhaled haloether anesthetics.
Organizational Affiliation:
Department of Anesthesiology & Critical Care, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA.