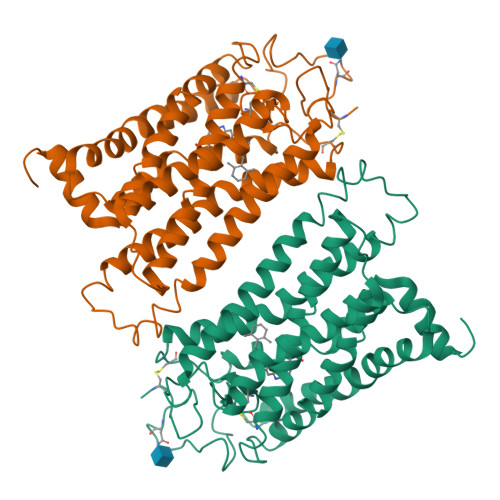
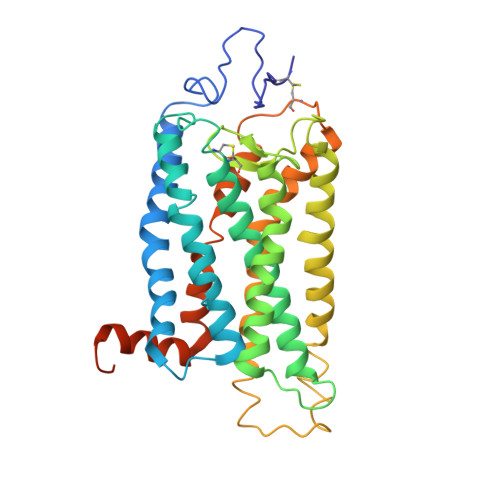
Crystal Structure of a Thermally Stable Rhodopsin Mutant.
Standfuss, J., Xie, G., Edwards, P.C., Burghammer, M., Oprian, D.D., Schertler, G.F.X.(2007) J Mol Biology 372: 1179
- PubMed: 17825322
- DOI: https://doi.org/10.1016/j.jmb.2007.03.007
- Primary Citation of Related Structures:
2J4Y - PubMed Abstract:
We determined the structure of the rhodopsin mutant N2C/D282C expressed in mammalian cells; the first structure of a recombinantly produced G protein-coupled receptor (GPCR). The mutant was designed to form a disulfide bond between the N terminus and loop E3, which allows handling of opsin in detergent solution and increases thermal stability of rhodopsin by 10 deg.C. It allowed us to crystallize a fully deglycosylated rhodopsin (N2C/N15D/D282C). N15 mutations are normally misfolding and cause retinitis pigmentosa in humans. Microcrystallographic techniques and a 5 microm X-ray beam were used to collect data along a single needle measuring 5 microm x 5 microm x 90 microm. The disulfide introduces only minor changes but fixes the N-terminal cap over the beta-sheet lid covering the ligand-binding site, a likely explanation for the increased stability. This work allows structural investigation of rhodopsin mutants and shows the problems encountered during structure determination of GPCRs and other mammalian membrane proteins.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Structural Studies, Hills Road, Cambridge CB2 2QH, UK.