The Solution Structure of the Adhesion Protein Bd37 from Babesia divergens Reveals Structural Homology with Eukaryotic Proteins Involved in Membrane Trafficking
Delbecq, S., Auguin, D., Yang, Y.S., Lohr, F., Arold, S., Schetters, T., Precigout, E., Gorenflot, A., Roumestand, C.(2007) J Mol Biology 375: 409-424
- PubMed: 18035372
- DOI: https://doi.org/10.1016/j.jmb.2007.08.019
- Primary Citation of Related Structures:
2JO7 - PubMed Abstract:
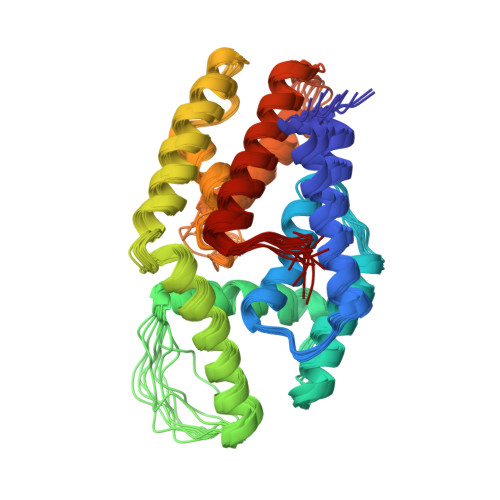
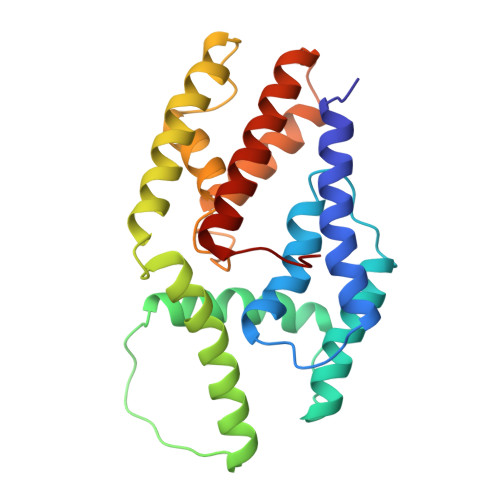
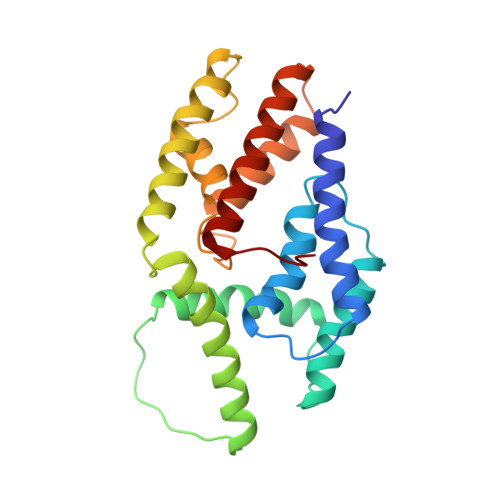
Babesia divergens is the Apicomplexa agent of the bovine babesiosis in Europe: this infection leads to growth and lactation decrease, so that economical losses due to this parasite are sufficient to require the development of a vaccine. The major surface antigen of B. divergens has been described as a 37 kDa protein glycosyl phosphatidyl inositol (GPI)-anchored at the surface of the merozoite. The immuno-prophylactic potential of Bd37 has been demonstrated, and we present here the high-resolution solution structure of the 27 kDa structured core of Bd37 (Delta-Bd37) using NMR spectroscopy. A model for the whole protein has been obtained using additional small angle X-ray scattering (SAXS) data. The knowledge of the 3D structure of Bd37 allowed the precise epitope mapping of antibodies on its surface. Interestingly, the geometry of Delta-Bd37 reveals an intriguing similarity with the exocyst subunit Exo84p C-terminal region, an eukaryotic protein that has a direct implication in vesicle trafficking. This strongly suggests that Apicomplexa have developed in parallel molecular machines similar in structure and function to the ones used for endo- and exocytosis in eukaryotic cells.
Organizational Affiliation:
DIMNP, Université Montpellier 1 et 2, CNRS, Laboratoire de Biologie Cellulaire et Moléculaire, ERT 1038, Faculté de Pharmacie BP 14491, Université de Montpellier I, 15 Avenue Charles Flahault, 34093 Montpellier Cedex 5, France.