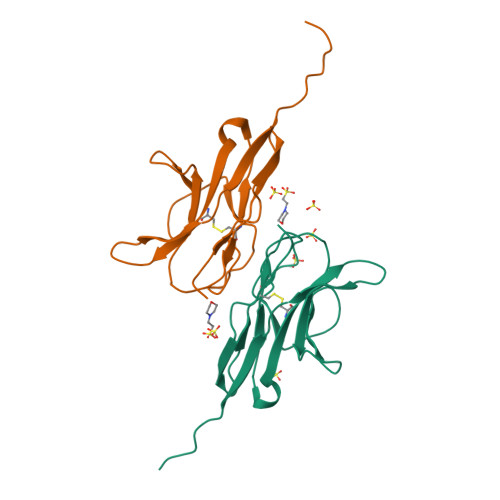
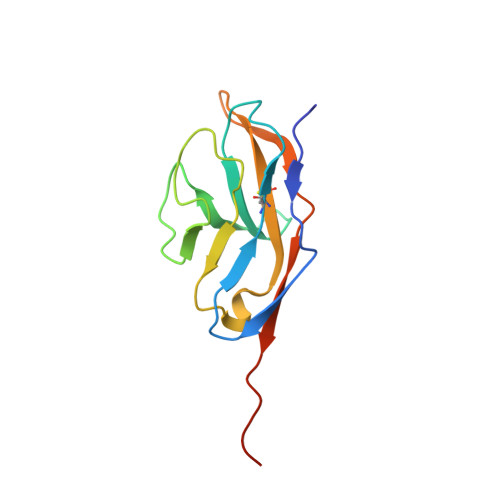
The Structure of the Macrophage Signal Regulatory Protein Alpha (Sirpalpha) Inhibitory Receptor Reveals a Binding Face Reminiscent of that Used by T Cell Receptors.
Hatherley, D., Harlos, K., Dunlop, D.C., Stuart, D.I., Barclay, A.N.(2007) J Biological Chem 282: 14567
- PubMed: 17369261
- DOI: https://doi.org/10.1074/jbc.M611511200
- Primary Citation of Related Structures:
2UV3 - PubMed Abstract:
Signal regulatory protein (SIRP) alpha is a membrane receptor that sends inhibitory signals to myeloid cells by engagement of CD47. The high resolution x-ray structure of the N-terminal ligand binding domain shows it to have a distinctive immunoglobulin superfamily V-like fold. Site-directed mutagenesis suggests that CD47 is bound at a surface involving the BC, FG, and DE loops, which distinguishes it from other immunoglobulin superfamily surface proteins that use the faces of the fold, but resembles antigen receptors. The SIRP interaction is confined to a single domain, and its use of an extended DE loop strengthens the similarity with T cell receptor binding and the suggestion that they are closely related in evolution. The employment of loops to form the CD47-binding surface provides a mechanism for small sequence changes to modulate binding specificity, explaining the different binding properties of SIRP family members.
Organizational Affiliation:
Sir William Dunn School of Pathology, University of Oxford, Oxford, UK.