Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling.
Hayashi, K., Tan, X., Zheng, N., Hatate, T., Kimura, Y., Kepinski, S., Nozaki, H.(2008) Proc Natl Acad Sci U S A 105: 5632-5637
- PubMed: 18391211
- DOI: https://doi.org/10.1073/pnas.0711146105
- Primary Citation of Related Structures:
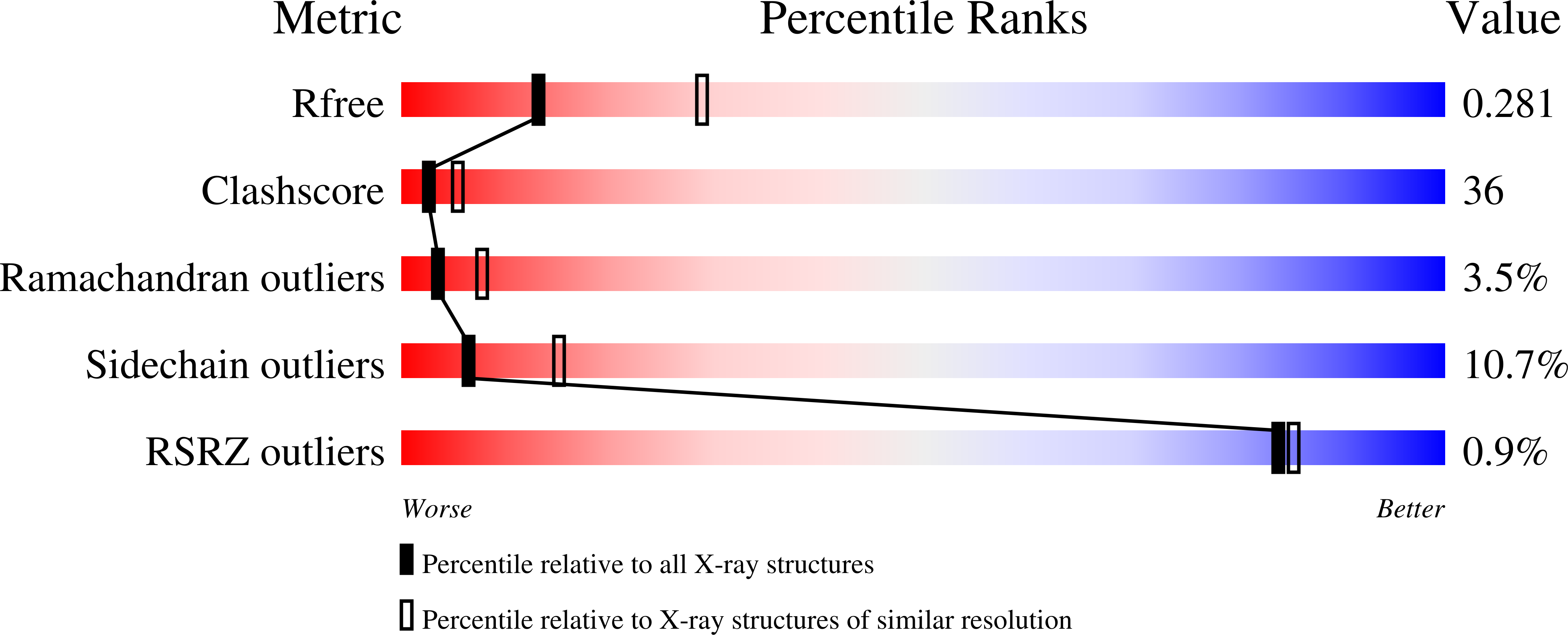
3C6N, 3C6O, 3C6P - PubMed Abstract:
The regulation of gene expression by the hormone auxin is a crucial mechanism in plant development. We have shown that the Arabidopsis F-box protein TIR1 is a receptor for auxin, and our recent structural work has revealed the molecular mechanism of auxin perception. TIR1 is the substrate receptor of the ubiquitin-ligase complex SCF(TIR1). Auxin binding enhances the interaction between TIR1 and its substrates, the Aux/IAA repressors, thereby promoting the ubiquitination and degradation of Aux/IAAs, altering the expression of hundreds of genes. TIR1 is the prototype of a new class of hormone receptor and the first example of an SCF ubiquitin-ligase modulated by a small molecule. Here, we describe the design, synthesis, and characterization of a series of auxin agonists and antagonists. We show these molecules are specific to TIR1-mediated events in Arabidopsis, and their mode of action in binding to TIR1 is confirmed by x-ray crystallographic analysis. Further, we demonstrate the utility of these probes for the analysis of TIR1-mediated auxin signaling in the moss Physcomitrella patens. Our work not only provides a useful tool for plant chemical biology but also demonstrates an example of a specific small-molecule inhibitor of F-box protein-substrate recruitment. Substrate recognition and subsequent ubiquitination by SCF-type ubiquitin ligases are central to many cellular processes in eukaryotes, and ubiquitin-ligase function is affected in several human diseases. Our work supports the idea that it may be possible to design small-molecule agents to modulate ubiquitin-ligase function therapeutically.
Organizational Affiliation:
Department of Biochemistry, Okayama University of Science, Okayama 700-0005, Japan.