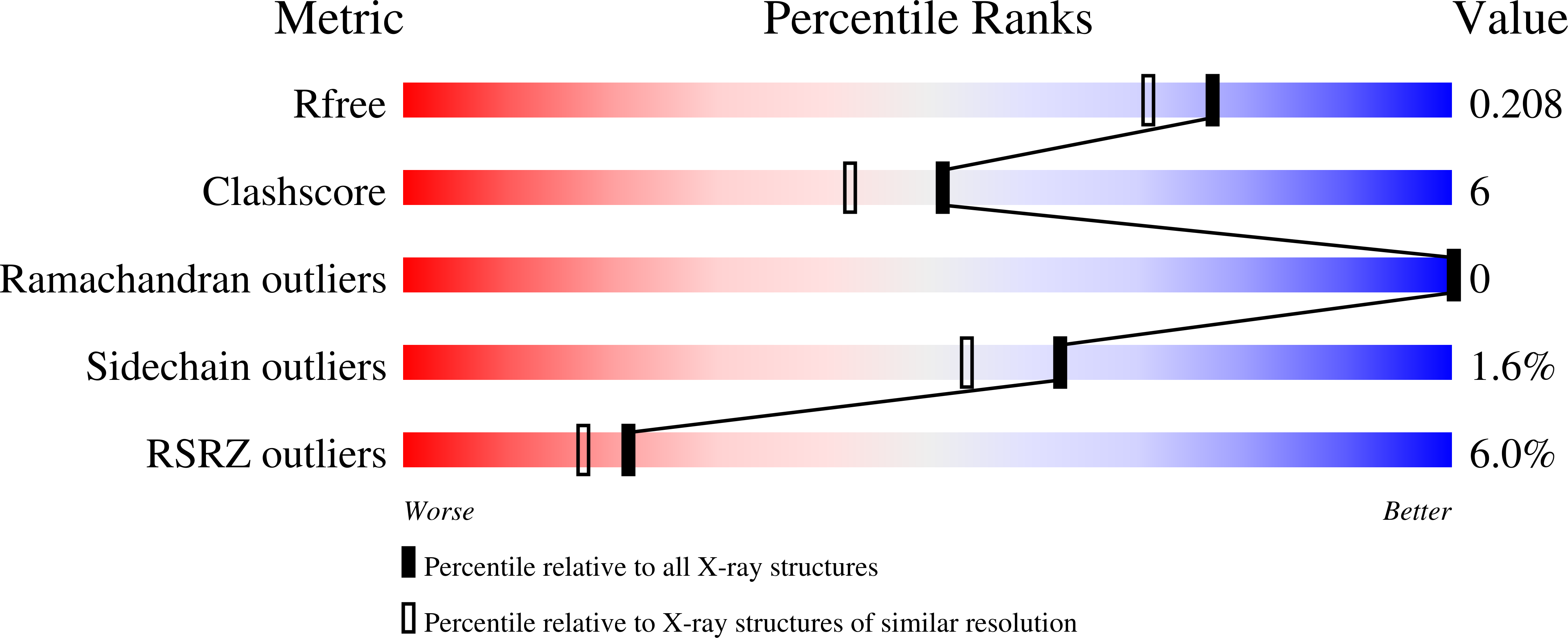
Structure elucidation and preliminary assessment of hydrolase activity of PqsE, the Pseudomonas quinolone signal (PQS) response protein.
Yu, S., Jensen, V., Seeliger, J., Feldmann, I., Weber, S., Schleicher, E., Haussler, S., Blankenfeldt, W.(2009) Biochemistry 48: 10298-10307
- PubMed: 19788310
- DOI: https://doi.org/10.1021/bi900123j
- Primary Citation of Related Structures:
2Q0I, 2Q0J, 3DH8 - PubMed Abstract:
In bacteria, the transcription of virulence genes is usually controlled by a cell density-dependent process known as "quorum sensing" (QS). QS relies on small diffusible signaling molecules that cross the bacterial cell wall and activate target transcription factors after a threshold concentration has been reached. Besides two hierarchical QS circuits based on N-acylhomoserine lactones, the human opportunistic pathogen Pseudomonas aeruginosa integrates a signaling system that depends on 2-heptyl-3-hydroxy-4-quinolone, termed "Pseudomonas quinolone signal" (PQS). PQS is produced from genes encoded in the pqs operon, which in addition to the biosynthetic enzymes PqsA-D contains a fifth gene, pqsE, that is not required for production of PQS but whose disruption leads to loss of signal transduction in several but not all pqs operon-dependent processes. PqsE was hence termed "PQS response protein", but its exact mechanism of action is unknown. We have determined the crystal structure of recombinant PqsE and show that it possesses a metallo-beta-lactamase fold with an Fe(II)Fe(III) center in the active site. A copurified ligand was assigned as benzoate and may indicate that PqsE exerts its regulatory effect by converting a chorismate-derived molecule. Further, PqsE was found to slowly hydrolyze phosphodiesters including single- and double-stranded DNA as well as mRNA and also the thioester S-(4-nitrobenzoyl)mercaptoethane. Higher activity was observed after incubation with Co(2+) and, to lesser entent, Mn(2+), suggesting that the Fe(II)Fe(III) center of recombinant PqsE may be an artifact of heterologous expression. A crystal complex of the E182A mutant with bis-pNPP was obtained and suggests a catalytic mechanism for hydrolysis.
Organizational Affiliation:
Department of Physical Biochemistry, Max-Planck-Institute of Molecular Physiology, 44227 Dortmund, Germany.