Development of a universal phosphorylated peptide-binding protein for simultaneous assay of kinases
Li, W., Bi, L., Wang, W., Li, Y., Zhou, Y., Wei, H., Jiang, T., Bai, L., Chen, Y., Zhang, Z., Yuan, X., Xiao, J., Zhang, X.-E.(2009) Biosens Bioelectron 24: 2871-2877
- PubMed: 19349157
- DOI: https://doi.org/10.1016/j.bios.2009.02.020
- Primary Citation of Related Structures:
3DPC - PubMed Abstract:
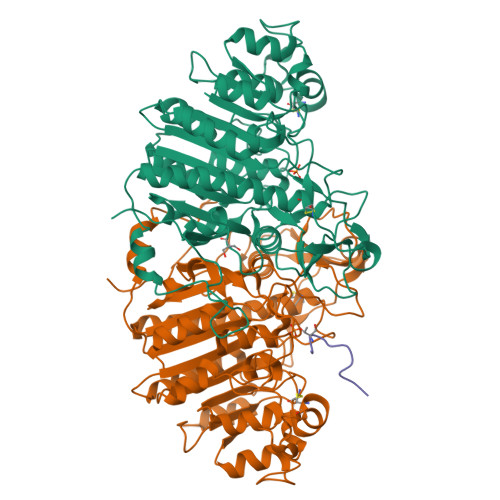
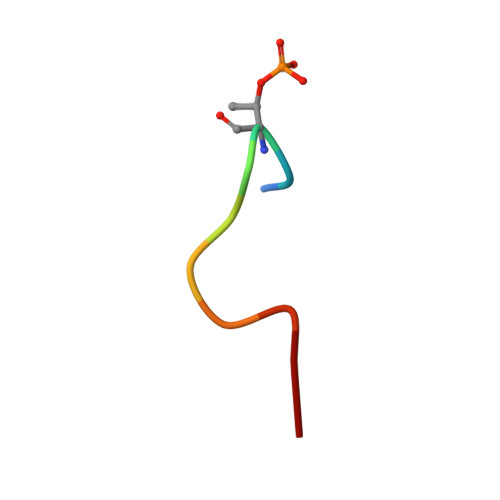
This study describes the development of a universal phosphorylated peptide-binding protein designed to simultaneously detect serine, threonine and tyrosine kinases. The Escherichia coli alkaline phosphatase (EAP) is a well-defined nonspecific phosphated monoesterase and Ser-, Thr- or Tyr-phosphorylated peptides served as substrates for EAP in preliminary experiments. Based on the known catalytic mechanism of EAP, the recombinant site-directed mutant EAP-S102L was generated, whose catalytic activity was blocked, but its binding ability was preserved. For EAP-S102L the catalytic rate constant, k(cat), was reduced by a factor of 1000, while the Michaelis-Menten constant, K(m), remained almost unchanged. Crystallographic analysis of the EAP-S102L/phophorylated peptide complex revealed that EAP-S102L could bind the phosphate group of the phosphorylated peptide but lacked nucleophilic attack potential which was essential for the catalytic ability of EAP. Finally, by combining the fluorescence-labeled EAP-S102L with non-phophorylated peptide chips, kinases could be detected from tumor cell samples. The recombinant EAP-S102L construct is perhaps the first functional binding protein derived from a native enzyme, illustrating how one single mutation tremendously alters protein function.
Organizational Affiliation:
State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan 430071, China.