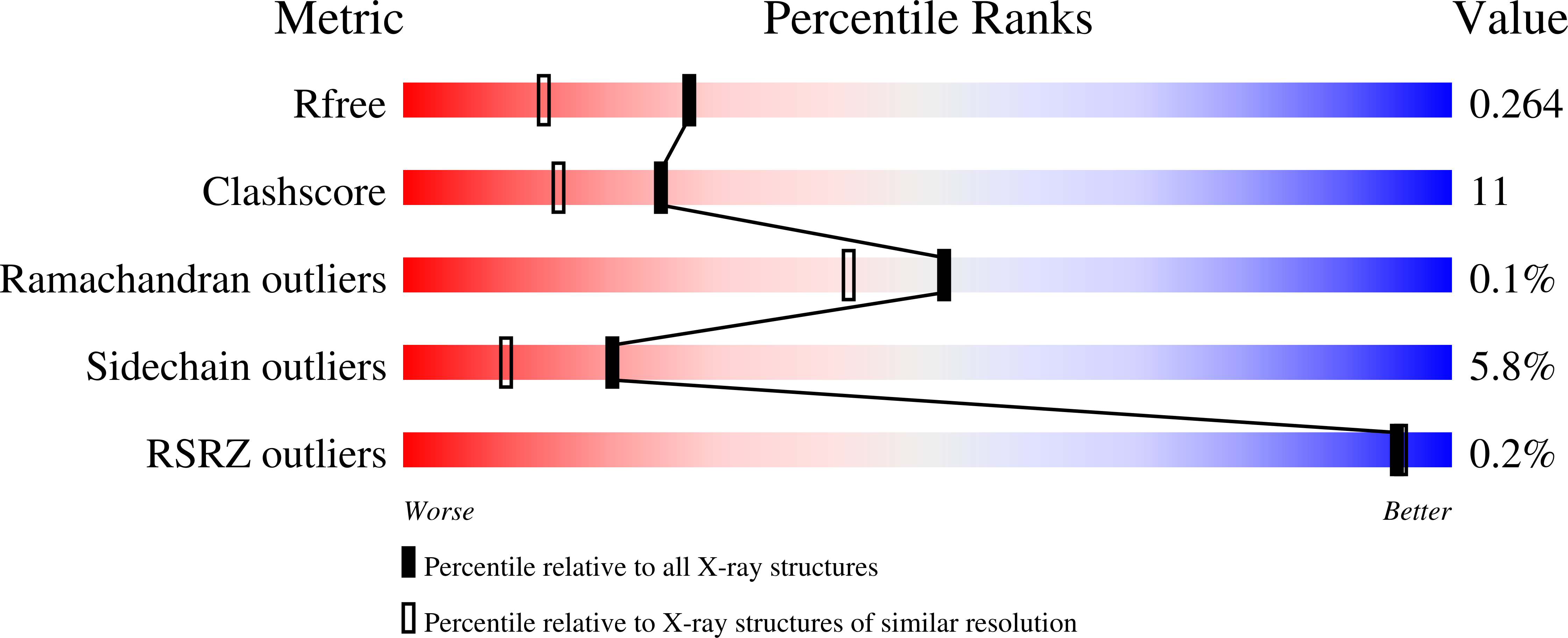
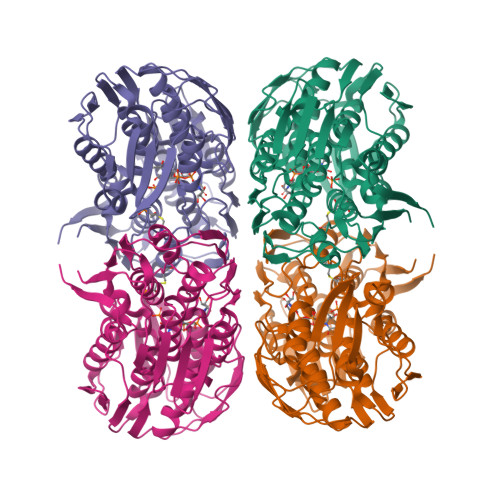
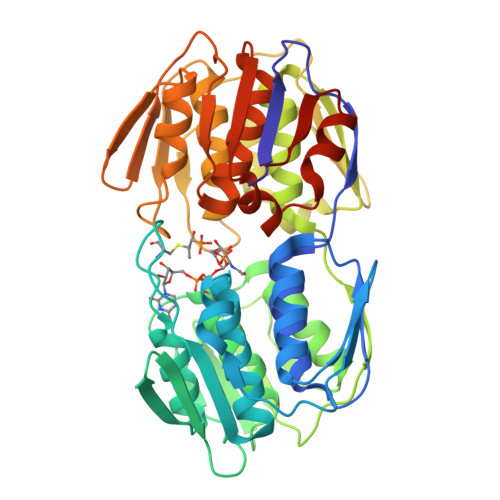
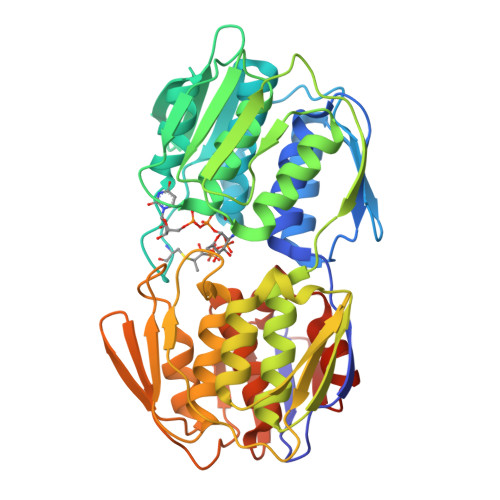
Structure of MurA (UDP-N-acetylglucosamine enolpyruvyl transferase) from Vibrio fischeri in complex with substrate UDP-N-acetylglucosamine and the drug fosfomycin.
Bensen, D.C., Rodriguez, S., Nix, J., Cunningham, M.L., Tari, L.W.(2012) Acta Crystallogr Sect F Struct Biol Cryst Commun 68: 382-385
- PubMed: 22505403
- DOI: https://doi.org/10.1107/S1744309112006720
- Primary Citation of Related Structures:
3VCY - PubMed Abstract:
The development of new antibiotics is necessitated by the rapid development of resistance to current therapies. UDP-N-acetylglucosamine enolpyruvyl transferase (MurA), which catalyzes the first committed step of bacterial peptidoglycan biosynthesis, is a prime candidate for therapeutic intervention. MurA is the target of the antibiotic fosfomycin, a natural product produced by Streptomyces. Despite possessing a high degree of sequence conservation with MurA enzymes from fosfomycin-susceptible organisms, recent microbiological studies suggest that MurA from Vibrio fischeri (VfiMurA) may confer fosfomycin resistance via a mechanism that is not yet understood. The crystal structure of VfiMurA in a ternary complex with the substrate UDP-N-acetylglucosamine (UNAG) and fosfomycin has been solved to a resolution of 1.93 Å. Fosfomycin is known to inhibit MurA by covalently binding to a highly conserved cysteine in the active site of the enzyme. A comparison of the title structure with the structure of fosfomycin-susceptible Haemophilus influenzae MurA (PDB entry 2rl2) revealed strikingly similar conformations of the mobile substrate-binding loop and clear electron density for a fosfomycin-cysteine adduct. Based on these results, there are no distinguishing sequence/structural features in VfiMurA that would translate to a diminished sensitivity to fosfomycin. However, VfiMurA is a robust crystallizer and shares high sequence identity with many clinically relevant bacterial pathogens. Thus, it would serve as an ideal system for use in the structure-guided optimization of new antibacterial agents.
Organizational Affiliation:
Structural Biology, Trius Therapeutics, 6310 Nancy Ridge Drive, Suite 101, San Diego, CA 92008, USA.