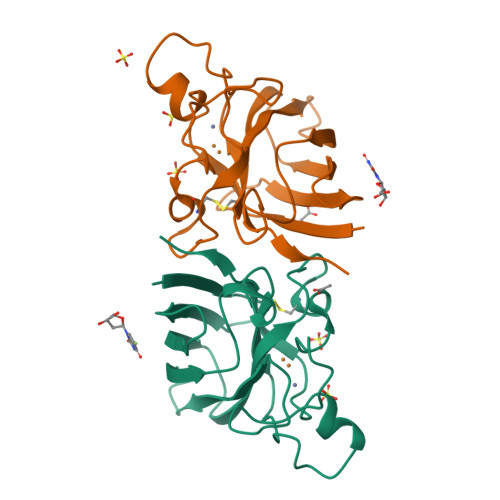
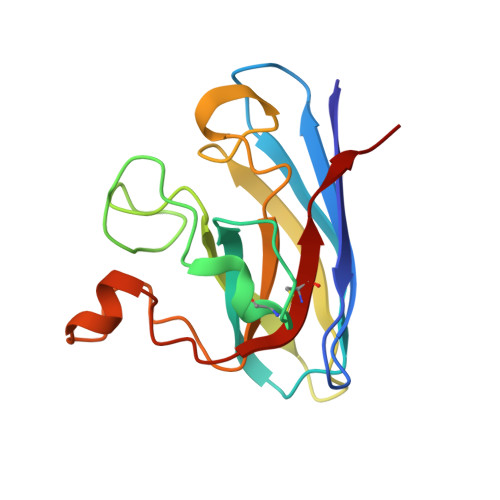
Ligand Binding and Aggregation of Pathogenic Sod1.
Wright, G.S.A., Antonyuk, S.V., Kershaw, N.M., Strange, R.W., Hasnain, S.S.(2013) Nat Commun 4: 1758
- PubMed: 23612299
- DOI: https://doi.org/10.1038/ncomms2750
- Primary Citation of Related Structures:
4A7S, 4A7T, 4A7U, 4A7V - PubMed Abstract:
Mutations in the gene encoding Cu/Zn superoxide dismutase-1 cause amyotrophic lateral sclerosis. Superoxide dismutase-1 mutations decrease protein stability and promote aggregation. The mutant monomer is thought to be an intermediate in the pathway from the superoxide dismutase-1 dimer to aggregate. Here we find that the monomeric copper-apo, zinc-holo protein is structurally perturbed and the apo-protein aggregates without reattainment of the monomer-dimer equilibrium. Intervention to stabilize the superoxide dismutase-1 dimer and inhibit aggregation is regarded as a potential therapeutic strategy. We describe protein-ligand interactions for two compounds, Isoproterenol and 5-fluorouridine, highlighted as superoxide dismutase-1 stabilizers. We find both compounds interact with superoxide dismutase-1 at a key region identified at the core of the superoxide dismutase-1 fibrillar aggregates, β-barrel loop II-strand 3, rather than the proposed dimer interface site. This illustrates the need for direct structural observations when developing compounds for protein-targeted therapeutics.
Organizational Affiliation:
Molecular Biophysics Group, Institute of Integrative Biology, Faculty of Health and Life Sciences, University of Liverpool, Liverpool L69 7ZB, UK.