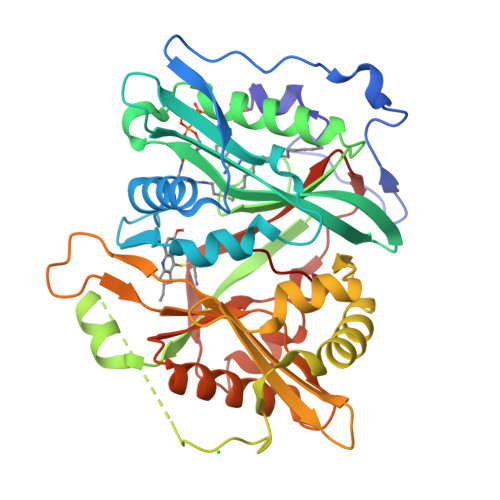
Discovery of Plasmodium Vivax N-Myristoyltransferase Inhibitors: Screening, Synthesis, and Structural Characterization of Their Binding Mode.
Goncalves, V., Brannigan, J.A., Whalley, D., Ansell, K.H., Saxty, B., Holder, A.A., Wilkinson, A.J., Tate, E.W., Leatherbarrow, R.J.(2012) J Med Chem 55: 3578
- PubMed: 22439843
- DOI: https://doi.org/10.1021/jm300040p
- Primary Citation of Related Structures:
4A95 - PubMed Abstract:
N-Myristoyltransferase (NMT) is a prospective drug target against parasitic protozoa. Herein we report the successful discovery of a series of Plasmodium vivax NMT inhibitors by high-throughput screening. A high-resolution crystal structure of the hit compound in complex with NMT was obtained, allowing understanding of its novel binding mode. A set of analogues was designed and tested to define the chemical groups relevant for activity and selectivity.
Organizational Affiliation:
Department of Chemistry, Imperial College London, London SW7 2AZ, United Kingdom.