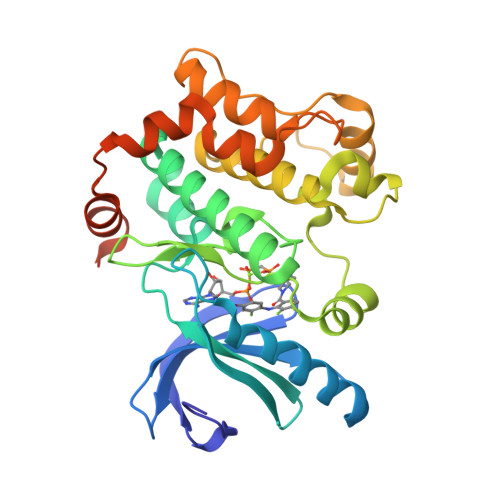
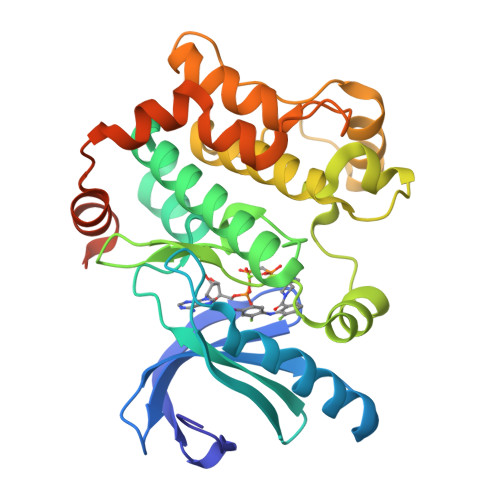
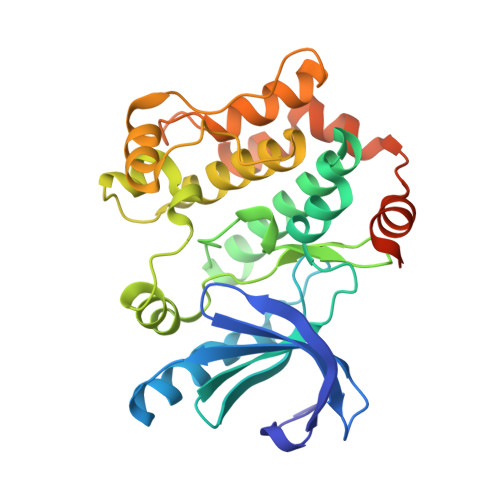
Novel Carboxamide-Based Allosteric Mek Inhibitors: Discovery and Optimization Efforts Toward Xl518 (Gdc-0973)
Rice, K.D., Aay, N., Anand, N.K., Blazey, C.M., Bowles, O.J., Bussenius, J., Costanzo, S., Curtis, J.K., Defina, S.C., Dubenko, L., Engst, S., Joshi, A.A., Kennedy, A.R., Kim, A.I., Koltun, E.S., Lougheed, J.C., Manalo, J.C.L., Martini, J.F., Nuss, J.M., Peto, C.J., Tsang, T.H., Yu, P., Johnston, S.(2012) ACS Med Chem Lett 3: 416
- PubMed: 24900486
- DOI: https://doi.org/10.1021/ml300049d
- Primary Citation of Related Structures:
4AN2, 4AN3, 4AN9, 4ANB - PubMed Abstract:
The ERK/MAP kinase cascade is a key mechanism subject to dysregulation in cancer and is constitutively activated or highly upregulated in many tumor types. Mutations associated with upstream pathway components RAS and Raf occur frequently and contribute to the oncogenic phenotype through activation of MEK and then ERK. Inhibitors of MEK have been shown to effectively block upregulated ERK/MAPK signaling in a range of cancer cell lines and have further demonstrated early evidence of efficacy in the clinic for the treatment of cancer. Guided by structural insight, a strategy aimed at the identification of an optimal diphenylamine-based MEK inhibitor with an improved metabolism and safety profile versus PD-0325901 led to the discovery of development candidate 1-({3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]phenyl}carbonyl)-3-[(2S)-piperidin-2-yl]azetidin-3-ol (XL518, GDC-0973) (1). XL518 exhibits robust in vitro and in vivo potency and efficacy in preclinical models with sustained duration of action and is currently in early stage clinical trials.
Organizational Affiliation:
Exelixis Inc. , 210 East Grand Avenue, South San Francisco, California 94080, United States.