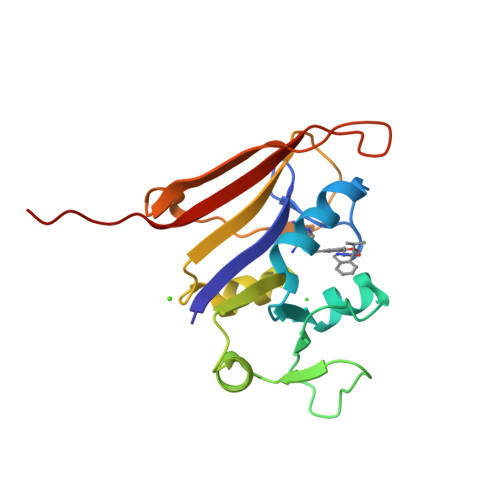
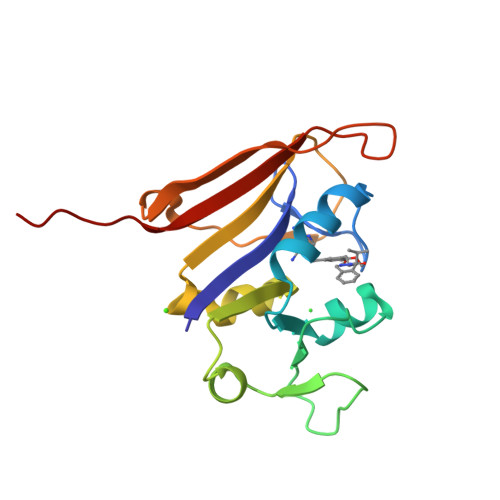
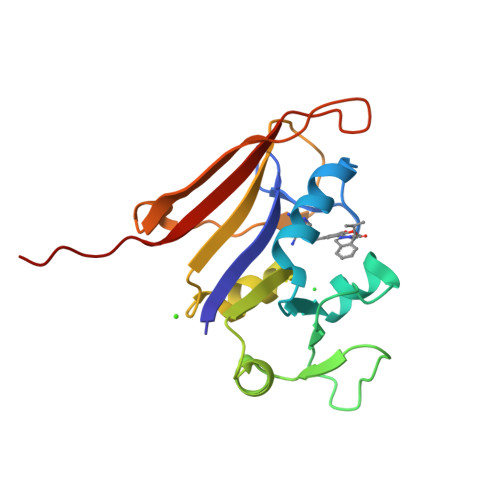
Structure-activity relationship for enantiomers of potent inhibitors of B. anthracis dihydrofolate reductase.
Bourne, C.R., Wakeham, N., Nammalwar, B., Tseitin, V., Bourne, P.C., Barrow, E.W., Mylvaganam, S., Ramnarayan, K., Bunce, R.A., Berlin, K.D., Barrow, W.W.(2013) Biochim Biophys Acta 1834: 46-52
- PubMed: 22999981
- DOI: https://doi.org/10.1016/j.bbapap.2012.09.001
- Primary Citation of Related Structures:
4ELB, 4ELE, 4ELF, 4ELG, 4ELH - PubMed Abstract:
Bacterial resistance to antibiotic therapies is increasing and new treatment options are badly needed. There is an overlap between these resistant bacteria and organisms classified as likely bioterror weapons. For example, Bacillus anthracis is innately resistant to the anti-folate trimethoprim due to sequence changes found in the dihydrofolate reductase enzyme. Development of new inhibitors provides an opportunity to enhance the current arsenal of anti-folate antibiotics while also expanding the coverage of the anti-folate class. We have characterized inhibitors of B. anthracis dihydrofolate reductase by measuring the K(i) and MIC values and calculating the energetics of binding. This series contains a core diaminopyrimidine ring, a central dimethoxybenzyl ring, and a dihydrophthalazine moiety. We have altered the chemical groups extended from a chiral center on the dihydropyridazine ring of the phthalazine moiety. The interactions for the most potent compounds were visualized by X-ray structure determination. We find that the potency of individual enantiomers is divergent with clear preference for the S-enantiomer, while maintaining a high conservation of contacts within the binding site. The preference for enantiomers seems to be predicated largely by differential interactions with protein residues Leu29, Gln30 and Arg53. These studies have clarified the activity of modifications and of individual enantiomers, and highlighted the role of the less-active R-enantiomer in effectively diluting the more active S-enantiomer in racemic solutions. This directly contributes to the development of new antimicrobials, combating trimethoprim resistance, and treatment options for potential bioterrorism agents.
Organizational Affiliation:
Department of Veterinary Pathobiology, Center for Veterinary Health Sciences, Oklahoma State University, Stillwater, OK 74078, USA. christina.bourne@okstate.edu