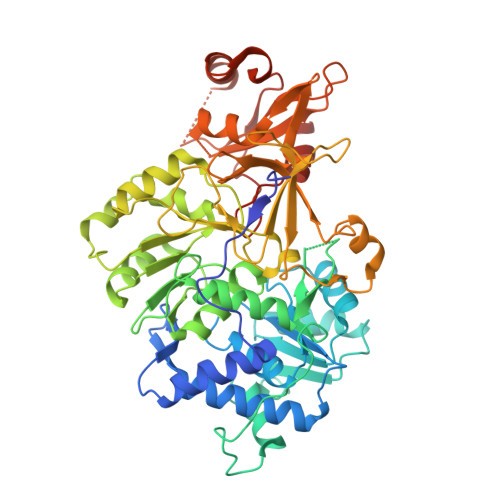
Crystal structure of firefly luciferase in a second catalytic conformation supports a domain alternation mechanism.
Sundlov, J.A., Fontaine, D.M., Southworth, T.L., Branchini, B.R., Gulick, A.M.(2012) Biochemistry 51: 6493-6495
- PubMed: 22852753
- DOI: https://doi.org/10.1021/bi300934s
- Primary Citation of Related Structures:
4G36, 4G37 - PubMed Abstract:
Beetle luciferases catalyze a two-step reaction that includes the initial adenylation of the luciferin substrate, followed by an oxidative decarboxylation that ultimately produces light. Evidence for homologous acyl-CoA synthetases supports a domain alternation catalytic mechanism in which these enzymes' C-terminal domain rotates by ~140° to adopt two conformations that are used to catalyze the two partial reactions. While many structures exist of acyl-CoA synthetases in both conformations, to date only biochemical evidence supports domain alternation with luciferase. We have determined the structure of a cross-linked luciferase enzyme that is trapped in the second conformation. This new structure supports the role of the second catalytic conformation and provides insights into the biochemical mechanism of the luciferase oxidative step.
Organizational Affiliation:
Hauptman-Woodward Institute, 700 Ellicott Street, Buffalo, NY 14203, USA.