Antibacterial drug leads targeting isoprenoid biosynthesis.
Zhu, W., Zhang, Y., Sinko, W., Hensler, M.E., Olson, J., Molohon, K.J., Lindert, S., Cao, R., Li, K., Wang, K., Wang, Y., Liu, Y.L., Sankovsky, A., de Oliveira, C.A., Mitchell, D.A., Nizet, V., McCammon, J.A., Oldfield, E.(2013) Proc Natl Acad Sci U S A 110: 123-128
- PubMed: 23248302
- DOI: https://doi.org/10.1073/pnas.1219899110
- Primary Citation of Related Structures:
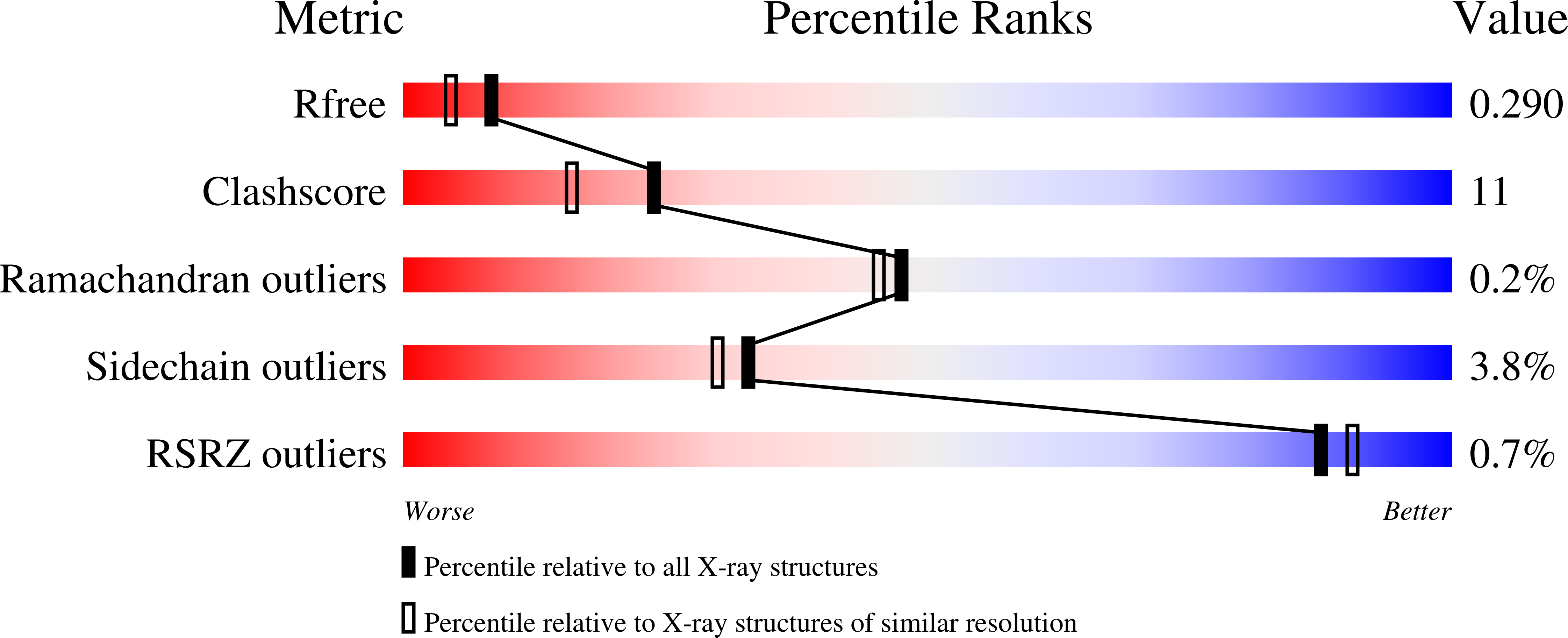
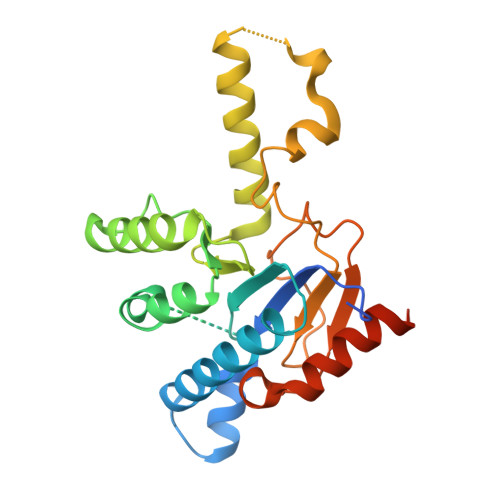
3SGT, 3SGV, 3SGX, 3SH0, 4H2J, 4H2M, 4H2O, 4H38, 4H3A, 4H3C, 4H8E - PubMed Abstract:
With the rise in resistance to antibiotics such as methicillin, there is a need for new drugs. We report here the discovery and X-ray crystallographic structures of 10 chemically diverse compounds (benzoic, diketo, and phosphonic acids, as well as a bisamidine and a bisamine) that inhibit bacterial undecaprenyl diphosphate synthase, an essential enzyme involved in cell wall biosynthesis. The inhibitors bind to one or more of the four undecaprenyl diphosphate synthase inhibitor binding sites identified previously, with the most active leads binding to site 4, outside the catalytic center. The most potent leads are active against Staphylococcus aureus [minimal inhibitory concentration (MIC)(90) ∼0.25 µg/mL], and one potently synergizes with methicillin (fractional inhibitory concentration index = 0.25) and is protective in a mouse infection model. These results provide numerous leads for antibacterial development and open up the possibility of restoring sensitivity to drugs such as methicillin, using combination therapies.
Organizational Affiliation:
Center for Biophysics and Computational Biology, University of Illinois, Urbana, IL 61801, USA.