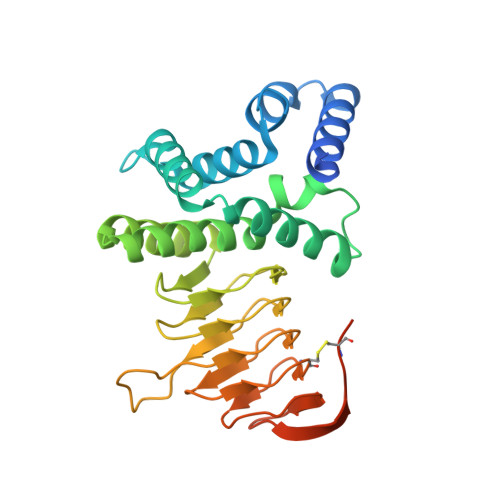
Crystal structure of serine acetyl transferase from Brucella abortus and its complex with coenzyme A.
Kumar, S., Kumar, N., Alam, N., Gourinath, S.(2014) Biochim Biophys Acta 1844: 1741-1748
- PubMed: 25058332
- DOI: https://doi.org/10.1016/j.bbapap.2014.07.009
- Primary Citation of Related Structures:
4HZC, 4HZD - PubMed Abstract:
Brucella abortus is the major cause of premature foetal abortion in cattle, can be transmitted from cattle to humans, and is considered a powerful biological weapon. De novo cysteine biosynthesis is one of the essential pathways reported in bacteria, protozoa, and plants. Serine acetyltransferase (SAT) initiates this reaction by catalyzing the formation of O-acetylserine (OAS) using l-serine and acetyl coenzyme A as substrates. Here we report kinetic and crystallographic studies of this enzyme from B. abortus. The kinetic studies indicate that cysteine competitively inhibits the binding of serine to B. abortus SAT (BaSAT) and noncompetitively inhibits the binding of acetyl coenzyme A. The crystal structures of BaSAT in its apo state and in complex with coenzyme A (CoA) were determined to 1.96Å and 1.87Å resolution, respectively. BaSAT was observed as a trimer in a size exclusion column; however, it was seen as a hexamer in dynamic light scattering (DLS) studies and in the crystal structure, indicating it may exist in both states. The complex structure shows coenzyme A bound to the C-terminal region, making mostly hydrophobic contacts from the center of the active site extending up to the surface of the protein. There is no conformational difference in the enzyme between the apo and the complexed states, indicating lock and key binding and the absence of an induced fit mechanism.
Organizational Affiliation:
School of Life Sciences, Jawaharlal Nehru University, New Delhi, Delhi 110067, India.