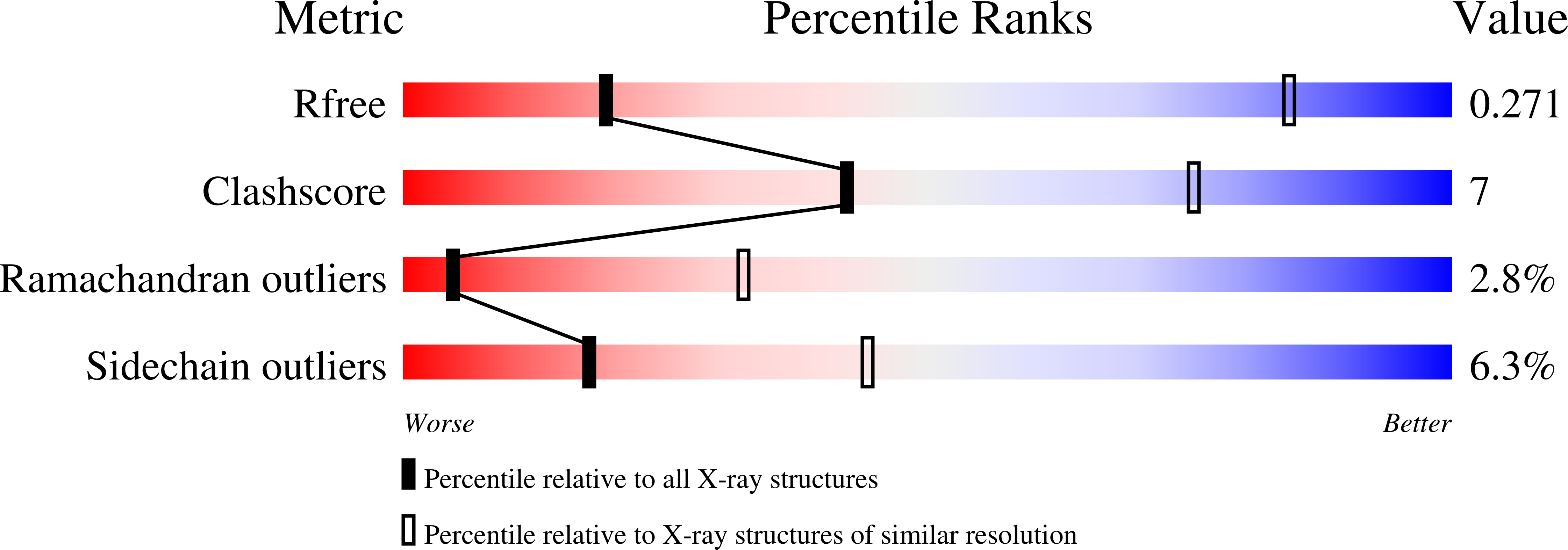
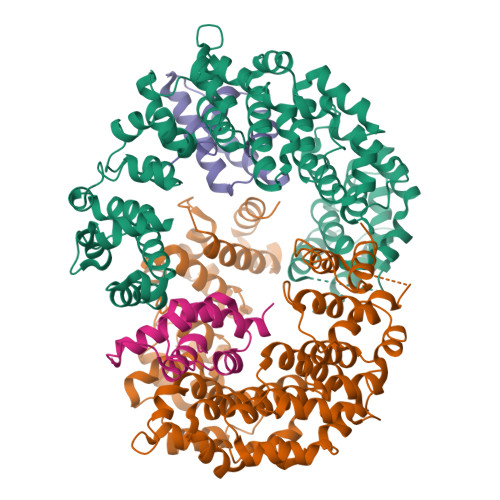
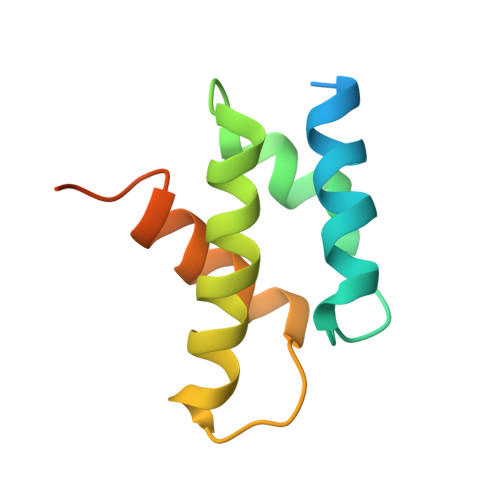
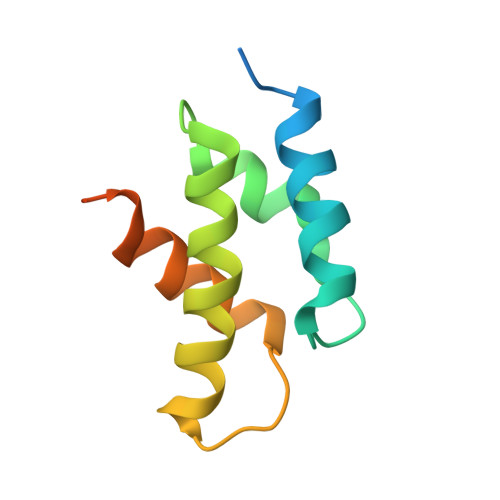
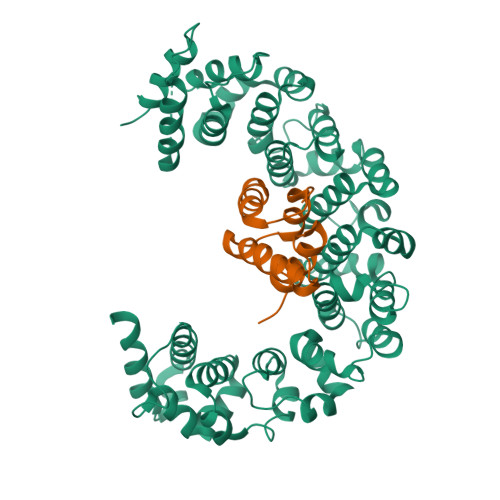
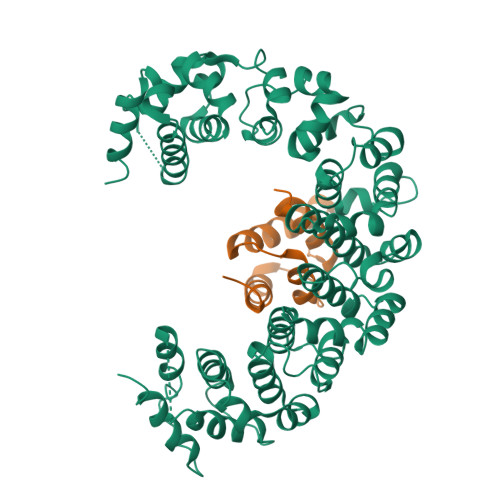
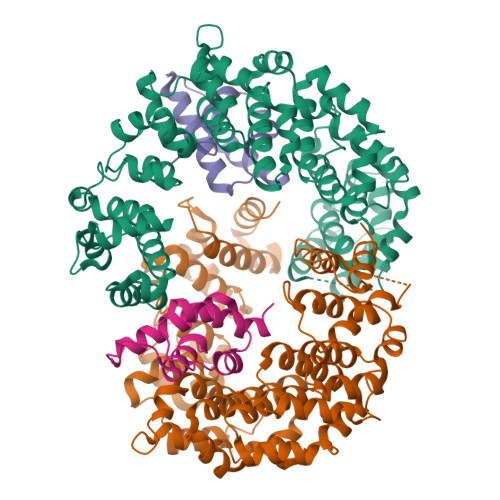
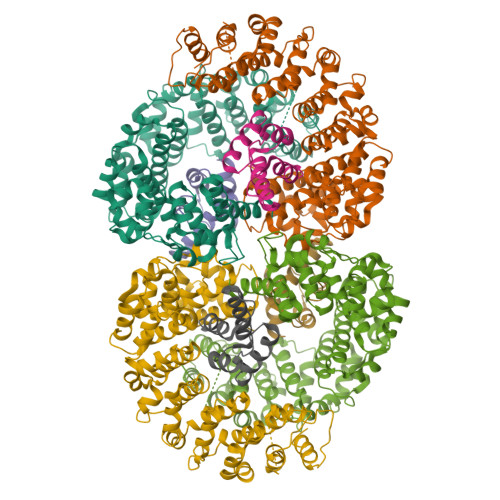
Reconfiguration of the proteasome during chaperone-mediated assembly.
Park, S., Li, X., Kim, H.M., Singh, C.R., Tian, G., Hoyt, M.A., Lovell, S., Battaile, K.P., Zolkiewski, M., Coffino, P., Roelofs, J., Cheng, Y., Finley, D.(2013) Nature 497: 512-516
- PubMed: 23644457
- DOI: https://doi.org/10.1038/nature12123
- Primary Citation of Related Structures:
4JPO - PubMed Abstract:
The proteasomal ATPase ring, comprising Rpt1-Rpt6, associates with the heptameric α-ring of the proteasome core particle (CP) in the mature proteasome, with the Rpt carboxy-terminal tails inserting into pockets of the α-ring. Rpt ring assembly is mediated by four chaperones, each binding a distinct Rpt subunit. Here we report that the base subassembly of the Saccharomyces cerevisiae proteasome, which includes the Rpt ring, forms a high-affinity complex with the CP. This complex is subject to active dissociation by the chaperones Hsm3, Nas6 and Rpn14. Chaperone-mediated dissociation was abrogated by a non-hydrolysable ATP analogue, indicating that chaperone action is coupled to nucleotide hydrolysis by the Rpt ring. Unexpectedly, synthetic Rpt tail peptides bound α-pockets with poor specificity, except for Rpt6, which uniquely bound the α2/α3-pocket. Although the Rpt6 tail is not visualized within an α-pocket in mature proteasomes, it inserts into the α2/α3-pocket in the base-CP complex and is important for complex formation. Thus, the Rpt-CP interface is reconfigured when the lid complex joins the nascent proteasome to form the mature holoenzyme.
Organizational Affiliation:
Department of Cell Biology, Harvard Medical School, 240 Longwood Avenue, Boston, Massachusetts 02115, USA.