Pre-steady-state Kinetic and Structural Analysis of Interaction of Methionine gamma-Lyase from Citrobacter freundii with Inhibitors.
Kuznetsov, N.A., Faleev, N.G., Kuznetsova, A.A., Morozova, E.A., Revtovich, S.V., Anufrieva, N.V., Nikulin, A.D., Fedorova, O.S., Demidkina, T.V.(2015) J Biological Chem 290: 671-681
- PubMed: 25398880
- DOI: https://doi.org/10.1074/jbc.M114.586511
- Primary Citation of Related Structures:
4OMA - PubMed Abstract:
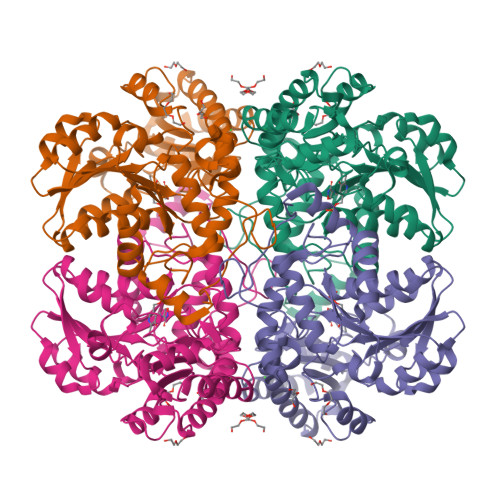
Methionine γ-lyase (MGL) catalyzes the γ-elimination of l-methionine and its derivatives as well as the β-elimination of l-cysteine and its analogs. These reactions yield α-keto acids and thiols. The mechanism of chemical conversion of amino acids includes numerous reaction intermediates. The detailed analysis of MGL interaction with glycine, l-alanine, l-norvaline, and l-cycloserine was performed by pre-steady-state stopped-flow kinetics. The structure of side chains of the amino acids is important both for their binding with enzyme and for the stability of the external aldimine and ketimine intermediates. X-ray structure of the MGL·l-cycloserine complex has been solved at 1.6 Å resolution. The structure models the ketimine intermediate of physiological reaction. The results elucidate the mechanisms of the intermediate interconversion at the stages of external aldimine and ketimine formation.
Organizational Affiliation:
From the Institute of Chemical Biology and Fundamental Medicine, Siberian Branch of the Russian Academy of Sciences, Novosibirsk 630090, the Department of Natural Sciences, Novosibirsk State University, Novosibirsk 630090.