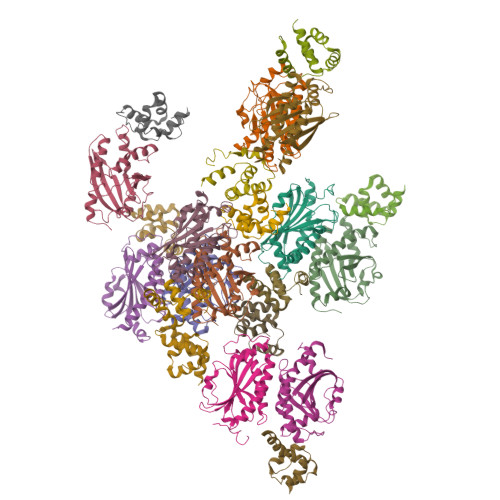
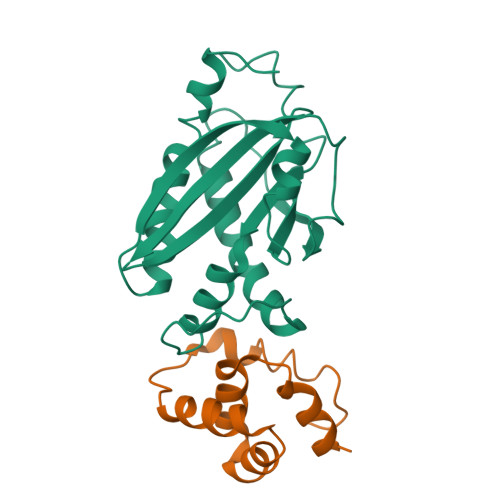
Assembly states of FliM and FliG within the flagellar switch complex.
Sircar, R., Borbat, P.P., Lynch, M.J., Bhatnagar, J., Beyersdorf, M.S., Halkides, C.J., Freed, J.H., Crane, B.R.(2015) J Mol Biology 427: 867-886
- PubMed: 25536293
- DOI: https://doi.org/10.1016/j.jmb.2014.12.009
- Primary Citation of Related Structures:
4QRM - PubMed Abstract:
At the base of the bacterial flagella, a cytoplasmic rotor (the C-ring) generates torque and reverses rotation sense in response to stimuli. The bulk of the C-ring forms from many copies of the proteins FliG, FliM, and FliN, which together constitute the switch complex. To help resolve outstanding issues regarding C-ring architecture, we have investigated interactions between FliM and FliG from Thermotoga maritima with X-ray crystallography and pulsed dipolar ESR spectroscopy (PDS). A new crystal structure of an 11-unit FliG:FliM complex produces a large arc with a curvature consistent with the dimensions of the C-ring. Previously determined structures along with this new structure provided a basis to test switch complex assembly models. PDS combined with mutational studies and targeted cross-linking reveal that FliM and FliG interact through their middle domains to form both parallel and antiparallel arrangements in solution. Residue substitutions at predicted interfaces disrupt higher-order complexes that are primarily mediated by contacts between the C-terminal domain of FliG and the middle domain of a neighboring FliG molecule. Spin separations among multi-labeled components fit a self-consistent model that agree well with electron microscopy images of the C-ring. An activated form of the response regulator CheY destabilizes the parallel arrangement of FliM molecules to perturb FliG alignment in a process that may reflect the onset of rotation switching. These data suggest a model of C-ring assembly in which intermolecular contacts among FliG domains provide a template for FliM assembly and cooperative transitions.
Organizational Affiliation:
Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853, USA.