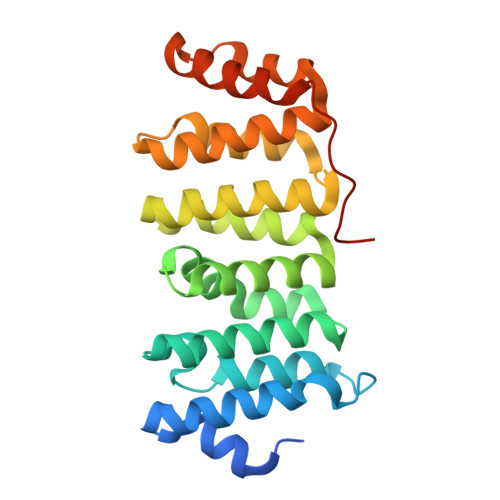
Drosophila melanogaster Mini Spindles TOG3 Utilizes Unique Structural Elements to Promote Domain Stability and Maintain a TOG1- and TOG2-like Tubulin-binding Surface.
Howard, A.E., Fox, J.C., Slep, K.C.(2015) J Biological Chem 290: 10149-10162
- PubMed: 25720490
- DOI: https://doi.org/10.1074/jbc.M114.633826
- Primary Citation of Related Structures:
4Y5J - PubMed Abstract:
Microtubule-associated proteins regulate microtubule (MT) dynamics spatially and temporally, which is essential for proper formation of the bipolar mitotic spindle. The XMAP215 family is comprised of conserved microtubule-associated proteins that use an array of tubulin-binding tumor overexpressed gene (TOG) domains, consisting of six (A-F) Huntingtin, elongation factor 3, protein phosphatase 2A, target of rapamycin (HEAT) repeats, to robustly increase MT plus-end polymerization rates. Recent work showed that TOG domains have differentially conserved architectures across the array, with implications for position-dependent TOG domain tubulin binding activities and function within the XMAP215 MT polymerization mechanism. Although TOG domains 1, 2, and 4 are well described, structural and mechanistic information characterizing TOG domains 3 and 5 is outstanding. Here, we present the structure and characterization of Drosophila melanogaster Mini spindles (Msps) TOG3. Msps TOG3 has two unique features as follows: the first is a C-terminal tail that stabilizes the ultimate four HEAT repeats (HRs), and the second is a unique architecture in HR B. Structural alignments of TOG3 with other TOG domain structures show that the architecture of TOG3 is most similar to TOG domains 1 and 2 and diverges from TOG4. Docking TOG3 onto recently solved Stu2 TOG1· and TOG2·tubulin complex structures suggests that TOG3 uses similarly conserved tubulin-binding intra-HEAT loop residues to engage α- and β-tubulin. This indicates that TOG3 has maintained a TOG1- and TOG2-like TOG-tubulin binding mode despite structural divergence. The similarity of TOG domains 1-3 and the divergence of TOG4 suggest that a TOG domain array with polarized structural diversity may play a key mechanistic role in XMAP215-dependent MT polymerization activity.
Organizational Affiliation:
From the Department of Biochemistry and Biophysics, Program in Molecular and Cellular Biophysics, and.