Mechanism of Inactivation of GABA Aminotransferase by (E)- and (Z)-(1S,3S)-3-Amino-4-fluoromethylenyl-1-cyclopentanoic Acid.
Lee, H., Le, H.V., Wu, R., Doud, E., Sanishvili, R., Kellie, J.F., Compton, P.D., Pachaiyappan, B., Liu, D., Kelleher, N.L., Silverman, R.B.(2015) ACS Chem Biol 10: 2087-2098
- PubMed: 26110556
- DOI: https://doi.org/10.1021/acschembio.5b00212
- Primary Citation of Related Structures:
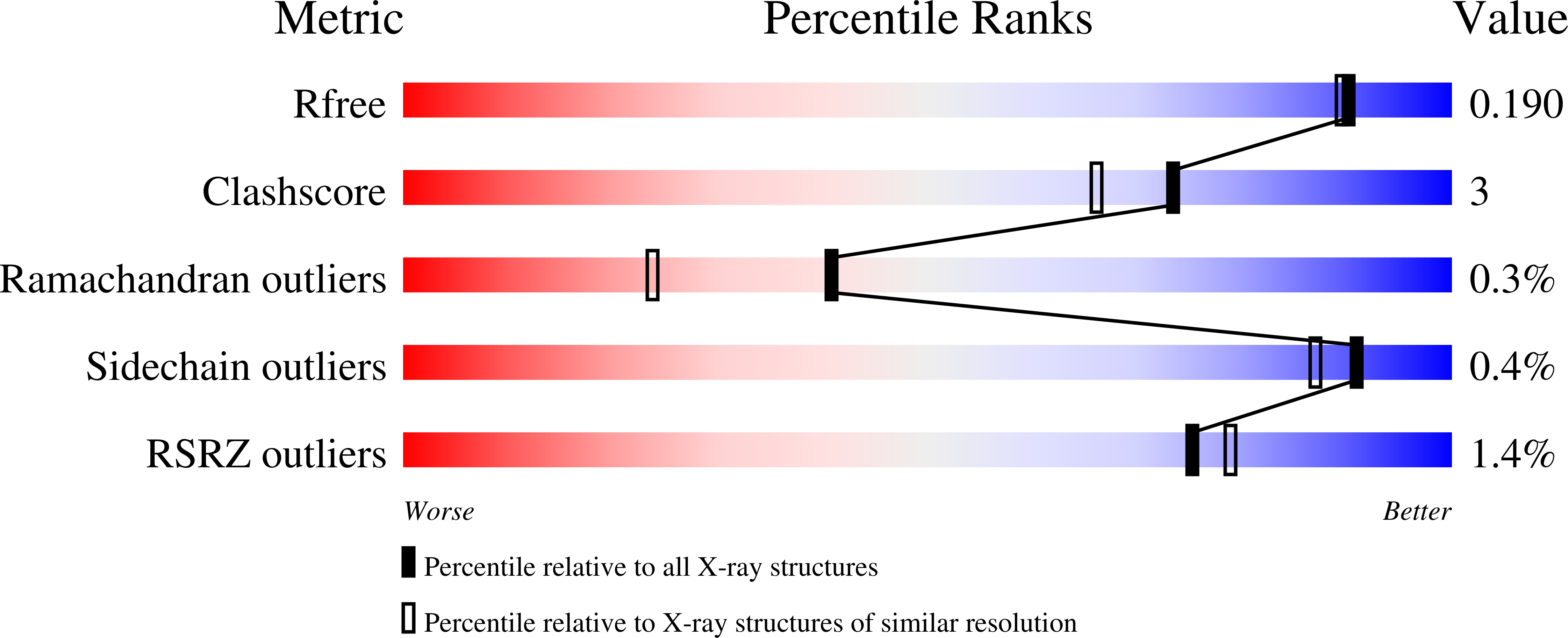
4ZSW, 4ZSY - PubMed Abstract:
When γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the mammalian central nervous system, falls below a threshold level, seizures occur. One approach to raise GABA concentrations is to inhibit GABA aminotransferase (GABA-AT), a pyridoxal 5'-phosphate-dependent enzyme that degrades GABA. We have previously developed (1S,3S)-3-amino-4-difluoromethylene-1-cyclopentanoic acid (CPP-115), which is 186 times more efficient in inactivating GABA-AT than vigabatrin, the only FDA-approved inactivator of GABA-AT. We also developed (E)- and (Z)-(1S,3S)-3-amino-4-fluoromethylenyl-1-cyclopentanoic acid (1 and 2, respectively), monofluorinated analogs of CPP-115, which are comparable to vigabatrin in inactivating GABA-AT. Here, we report the mechanism of inactivation of GABA-AT by 1 and 2. Both produce a metabolite that induces disruption of the Glu270-Arg445 salt bridge to accommodate interaction between the metabolite formyl group and Arg445. This is the second time that Arg445 has interacted with a ligand and is involved in GABA-AT inactivation, thereby confirming the importance of Arg445 in future inactivator design.
Organizational Affiliation:
Departments of Chemistry and Molecular Biosciences, Chemistry of Life Processes Institute, and the Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 60208, United States.