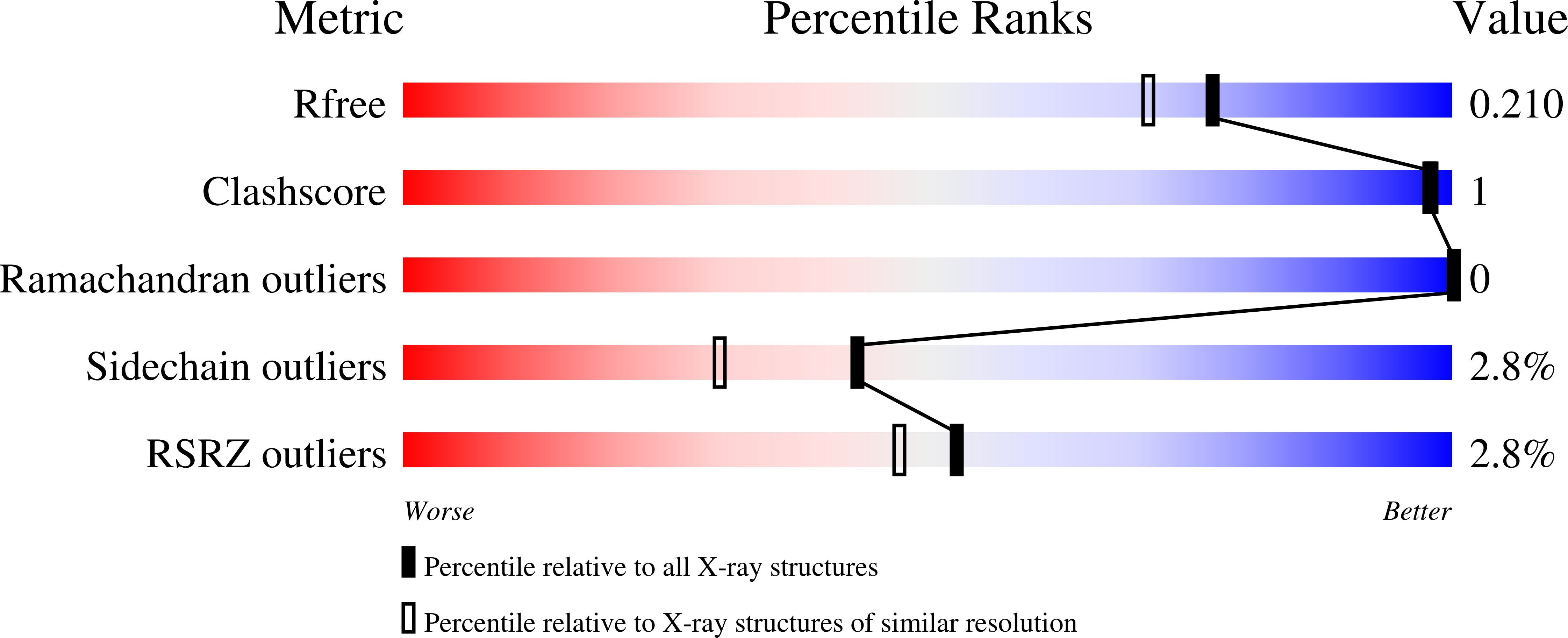
Structure of the Dioxygenase AsqJ: Mechanistic Insights into a One-Pot Multistep Quinolone Antibiotic Biosynthesis.
Brauer, A., Beck, P., Hintermann, L., Groll, M.(2016) Angew Chem Int Ed Engl 55: 422-426
- PubMed: 26553478
- DOI: https://doi.org/10.1002/anie.201507835
- Primary Citation of Related Structures:
5DAP, 5DAQ, 5DAV, 5DAW, 5DAX - PubMed Abstract:
Multienzymatic cascades are responsible for the biosynthesis of natural products and represent a source of inspiration for synthetic chemists. The Fe(II)/α-ketoglutarate-dependent dioxygenase AsqJ from Aspergillus nidulans is outstanding because it stereoselectively catalyzes both a ferryl-induced desaturation reaction and epoxidation on a benzodiazepinedione. Interestingly, the enzymatically formed spiro epoxide spring-loads the 6,7-bicyclic skeleton for non-enzymatic rearrangement into the 6,6-bicyclic scaffold of the quinolone alkaloid 4'-methoxyviridicatin. Herein, we report different crystal structures of the protein in the absence and presence of synthesized substrates, surrogates, and intermediates that mimic the various stages of the reaction cycle of this exceptional dioxygenase.
Organizational Affiliation:
Center for Integrated Protein Science Munich (CIPSM), Department of Chemistry, Technische Universität München, Lichtenbergstraße 4, 85748 Garching (Germany).