Gram-negative trimeric porins have specific LPS binding sites that are essential for porin biogenesis.
Arunmanee, W., Pathania, M., Solovyova, A.S., Le Brun, A.P., Ridley, H., Basle, A., van den Berg, B., Lakey, J.H.(2016) Proc Natl Acad Sci U S A 113: E5034-E5043
- PubMed: 27493217
- DOI: https://doi.org/10.1073/pnas.1602382113
- Primary Citation of Related Structures:
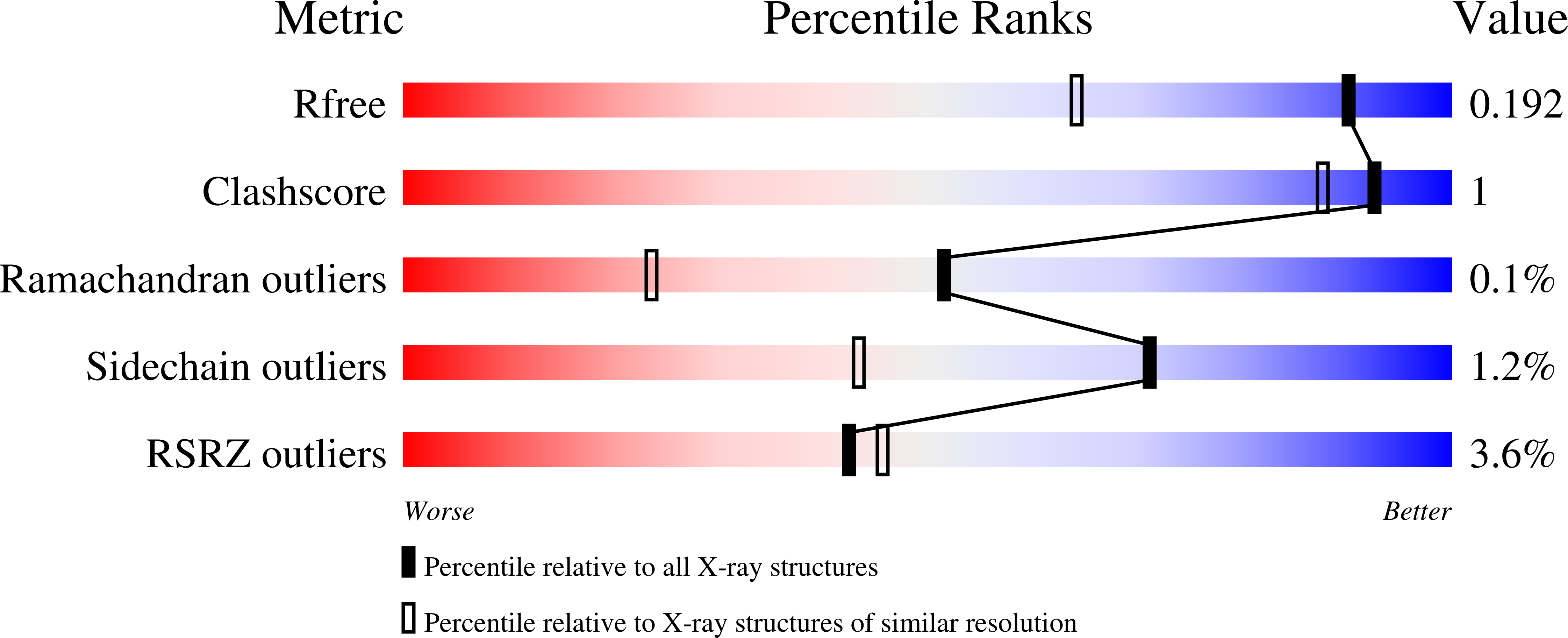
5FVN - PubMed Abstract:
The outer membrane (OM) of gram-negative bacteria is an unusual asymmetric bilayer with an external monolayer of lipopolysaccharide (LPS) and an inner layer of phospholipids. The LPS layer is rigid and stabilized by divalent cation cross-links between phosphate groups on the core oligosaccharide regions. This means that the OM is robust and highly impermeable to toxins and antibiotics. During their biogenesis, OM proteins (OMPs), which function as transporters and receptors, must integrate into this ordered monolayer while preserving its impermeability. Here we reveal the specific interactions between the trimeric porins of Enterobacteriaceae and LPS. Isolated porins form complexes with variable numbers of LPS molecules, which are stabilized by calcium ions. In earlier studies, two high-affinity sites were predicted to contain groups of positively charged side chains. Mutation of these residues led to the loss of LPS binding and, in one site, also prevented trimerization of the porin, explaining the previously observed effect of LPS mutants on porin folding. The high-resolution X-ray crystal structure of a trimeric porin-LPS complex not only helps to explain the mutagenesis results but also reveals more complex, subtle porin-LPS interactions and a bridging calcium ion.
Organizational Affiliation:
Institute for Cell and Molecular Biosciences, Newcastle University, Newcastle upon Tyne NE2 4HH, United Kingdom;