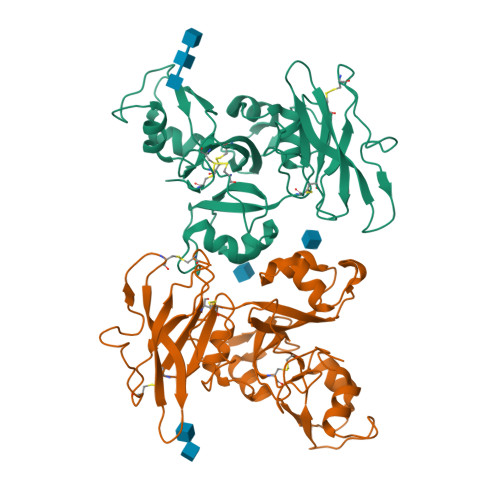
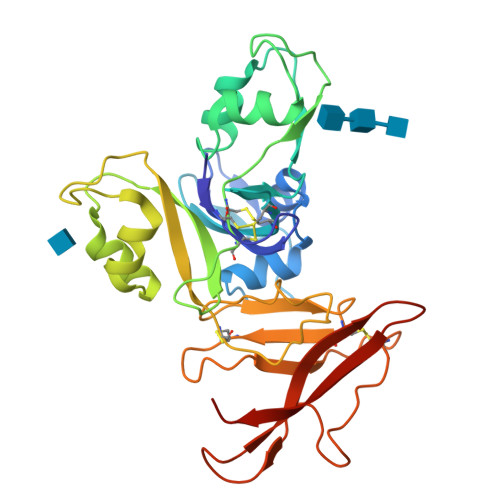
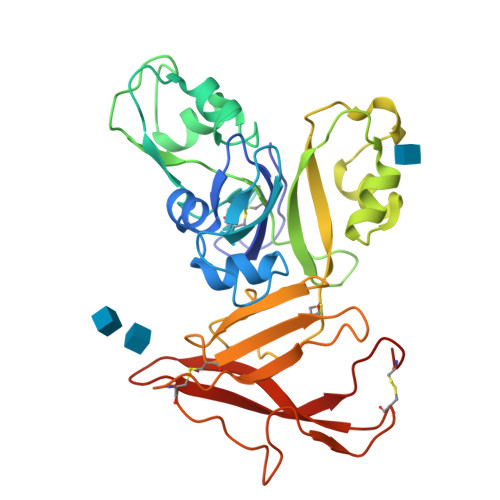
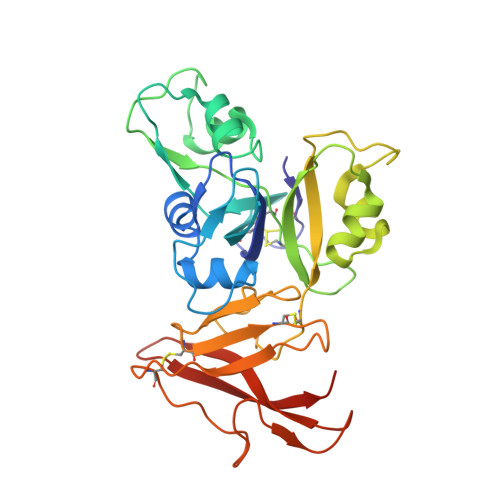
Molecular Mechanism for Fungal Cell Wall Recognition by Rice Chitin Receptor OsCEBiP
Liu, S.M., Wang, J.Z., Han, Z., Gong, X., Zhang, H., Chai, J.J.(2016) Structure 24: 1192-1200
- PubMed: 27238968
- DOI: https://doi.org/10.1016/j.str.2016.04.014
- Primary Citation of Related Structures:
5JCD, 5JCE - PubMed Abstract:
Chitin is the major component of fungal cell wall and serves as a molecular pattern that can be recognized by the receptor OsCEBiP in rice, a lysine motif (LysM) receptor-like protein (RLP), to trigger immune responses. The molecular mechanisms underlying chitin recognition remain elusive. Here we report the crystal structures of the ectodomain of OsCEBiP (OsCEBiP-ECD) in free and chitin-bound forms. The structures reveal that OsCEBiP-ECD contains three tandem LysMs followed by a novel structure fold of cysteine-rich domain. The structures showed that chitin binding induces no striking conformational changes in OsCEBiP. Structural comparison among N-acetylglucosamine (NAG) oligomer-bound LysMs revealed a highly conserved recognition mechanism, which is expected to facilitate study of other LysM-containing proteins for their NAG binding. Modeling study showed that chitin induces OsCEBiP homodimerization in a "sliding mode". Our data provide insights into rice chitin receptor-mediated immunity triggered by fungal cell wall.
Organizational Affiliation:
Ministry of Education Key Laboratory of Protein Science, Center for Structural Biology, School of Life Sciences, Tsinghua-Peking Joint Center for Life Sciences, Tsinghua University, Beijing 100084, China.