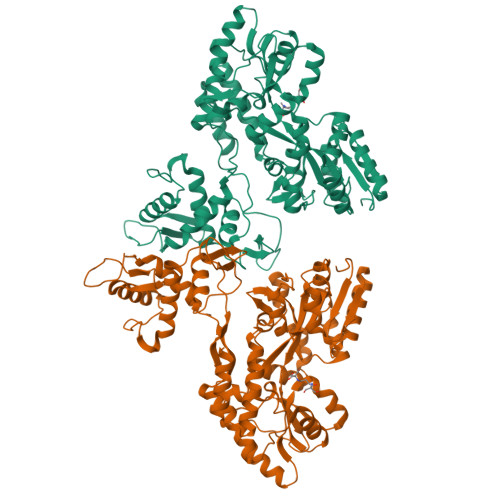
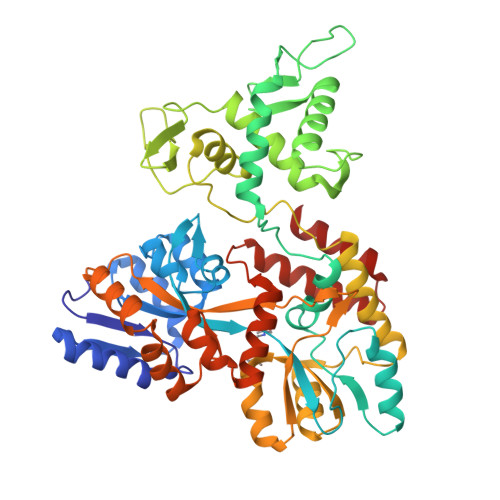
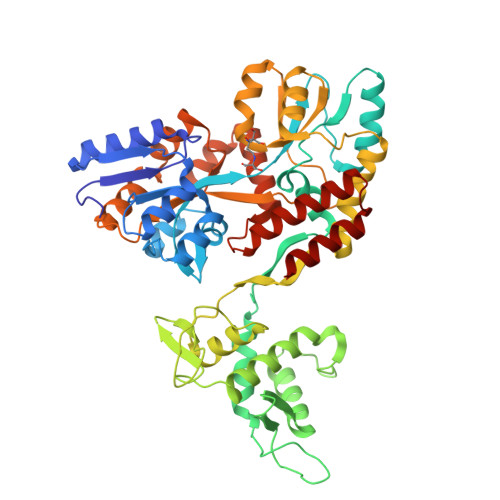
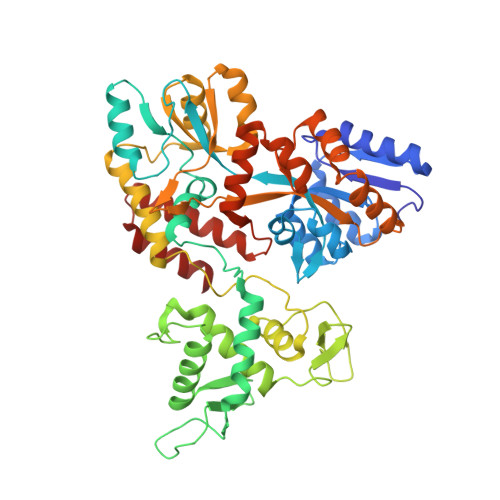
Characterization of a polypeptide-binding site in the DEAD Motor of the SecA ATPase.
Khalili Yazdi, A., Namjoshi, S., Hackett, J., Ghonaim, N., Shilton, B.H.(2017) FEBS Lett 591: 3378-3390
- PubMed: 28862749
- DOI: https://doi.org/10.1002/1873-3468.12832
- Primary Citation of Related Structures:
5K94 - PubMed Abstract:
We coupled peptides from a CNBr digest of signal-sequenceless maltose-binding protein (MBP) to a surface plasmon resonance chip. SecA-N95, SecA-N68, and SecA-DM (which consists of only the DEAD Motor domains NBD1 and NBD2) bound to the immobilized peptides; ADP weakened the binding. SecA-DM, which lacks the 'preprotein cross-linking domain' (PPXD), displayed the most extensive binding, while an MBP-PPXD chimera showed no binding, demonstrating that the PPXD does not contribute to the binding. We characterized the sequence specificity using oriented peptide libraries; these results enabled synthesis of a 20-residue peptide that was used to recapitulate the results obtained with MBP-derived peptides. This study shows that there is a promiscuous and nucleotide-modulated peptide-binding site in the DEAD Motor domains of SecA.
Organizational Affiliation:
Department of Biochemistry, University of Western Ontario, London, Canada.