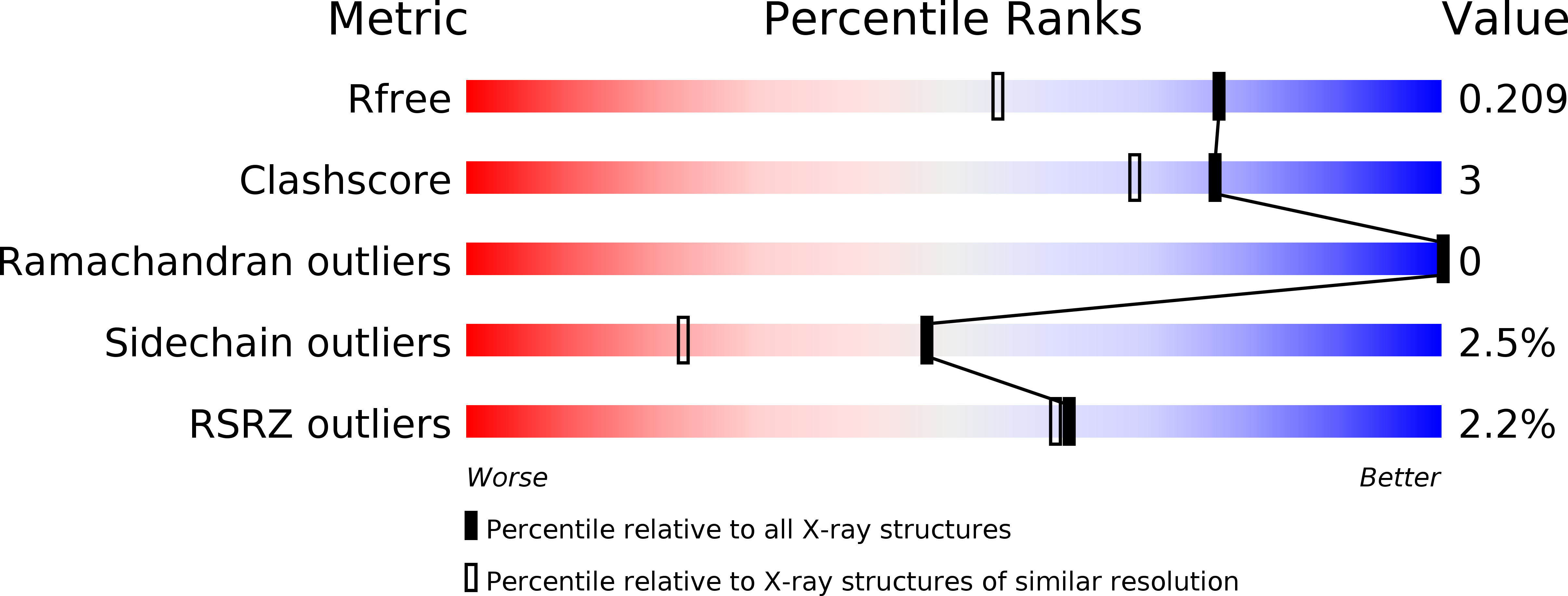
Electrostatic Tuning of the Ligand Binding Mechanism by Glu27 in Nitrophorin 7.
Abbruzzetti, S., Allegri, A., Bidon-Chanal, A., Ogata, H., Soavi, G., Cerullo, G., Bruno, S., Montali, C., Luque, F.J., Viappiani, C.(2018) Sci Rep 8: 10855-10855
- PubMed: 30022039
- DOI: https://doi.org/10.1038/s41598-018-29182-3
- Primary Citation of Related Structures:
5M6J, 5M6K - PubMed Abstract:
Nitrophorins (NP) 1-7 are NO-carrying heme proteins found in the saliva of the blood-sucking insect Rhodnius prolixus. The isoform NP7 displays peculiar properties, such as an abnormally high isoelectric point, the ability to bind negatively charged membranes, and a strong pH sensitivity of NO affinity. A unique trait of NP7 is the presence of Glu in position 27, which is occupied by Val in other NPs. Glu27 appears to be important for tuning the heme properties, but its influence on the pH-dependent NO release mechanism, which is assisted by a conformational change in the AB loop, remains unexplored. Here, in order to gain insight into the functional role of Glu27, we examine the effect of Glu27 → Val and Glu27 → Gln mutations on the ligand binding kinetics using CO as a model. The results reveal that annihilation of the negative charge of Glu27 upon mutation reduces the pH sensitivity of the ligand binding rate, a process that in turn depends on the ionization of Asp32. We propose that Glu27 exerts a through-space electrostatic action on Asp32, which shifts the pKa of the latter amino acid towards more acidic values thus reducing the pH sensitivity of the transition between open and closed states.
Organizational Affiliation:
Dipartimento di Scienze Matematiche, Fisiche e Informatiche, Università degli Studi di Parma, Parco Area delle Scienze 7/A, 43124, Parma, Italy. stefania.abbruzzetti@unipr.it.