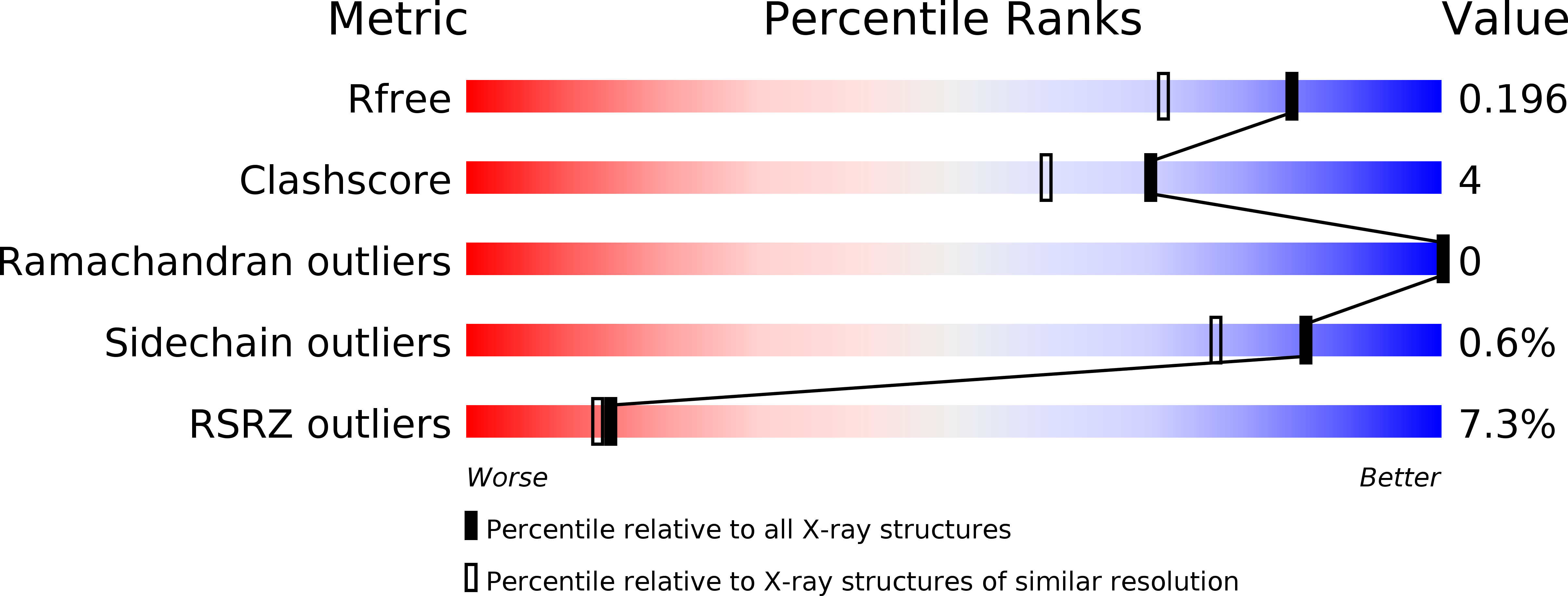
Design of an allosterically modulated doxycycline and doxorubicin drug-binding protein.
Schmidt, K., Gardill, B.R., Kern, A., Kirchweger, P., Borsch, M., Muller, Y.A.(2018) Proc Natl Acad Sci U S A 115: 5744-5749
- PubMed: 29760101
- DOI: https://doi.org/10.1073/pnas.1716666115
- Primary Citation of Related Structures:
5OM2, 5OM3, 5OM5, 5OM6, 5OM7, 5OM8, 6FTP - PubMed Abstract:
The allosteric interplay between distant functional sites present in a single protein provides for one of the most important regulatory mechanisms in biological systems. While the design of ligand-binding sites into proteins remains challenging, this holds even truer for the coupling of a newly engineered binding site to an allosteric mechanism that regulates the ligand affinity. Here it is shown how computational design algorithms enabled the introduction of doxycycline- and doxorubicin-binding sites into the serine proteinase inhibitor (serpin) family member α1-antichymotrypsin. Further engineering allowed exploitation of the proteinase-triggered serpin-typical S-to-R transition to modulate the ligand affinities. These design variants follow strategies observed in naturally occurring plasma globulins that allow for the targeted delivery of hormones in the blood. By analogy, we propose that the variants described in the present study could be further developed to allow for the delivery of the antibiotic doxycycline and the anticancer compound doxorubicin to tissues/locations that express specific proteinases, such as bacterial infection sites or tumor cells secreting matrix metalloproteinases.
Organizational Affiliation:
Department of Biotechnology, Friedrich-Alexander University Erlangen-Nuremberg, 91054 Erlangen, Germany.