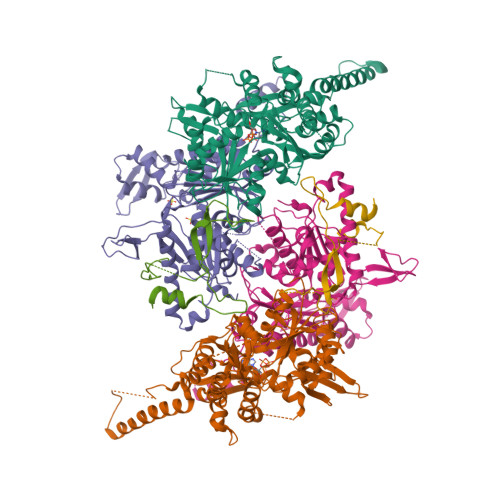
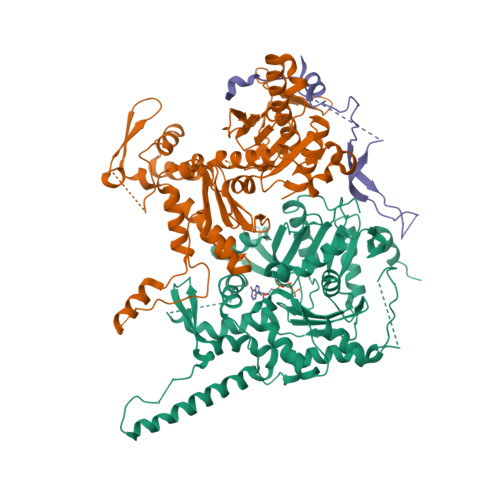
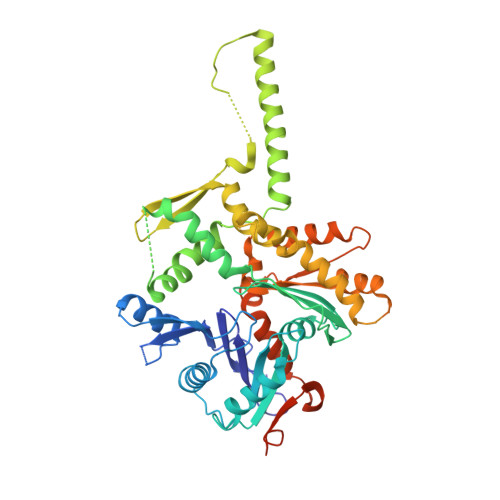
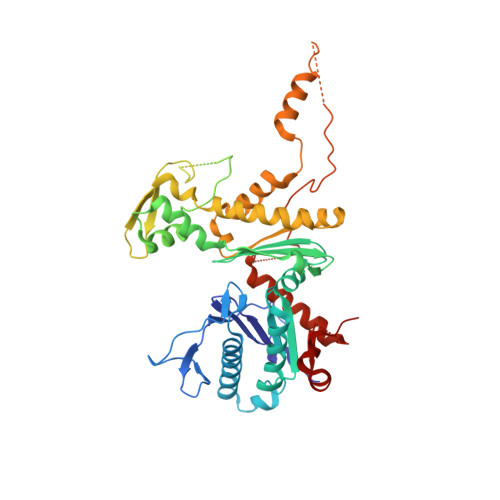
Actin-related proteins regulate the RSC chromatin remodeler by weakening intramolecular interactions of the Sth1 ATPase.
Turegun, B., Baker, R.W., Leschziner, A.E., Dominguez, R.(2018) Commun Biol 1
- PubMed: 29809203
- DOI: https://doi.org/10.1038/s42003-017-0002-6
- Primary Citation of Related Structures:
5TGC - PubMed Abstract:
The catalytic subunits of SWI/SNF-family and INO80-family chromatin remodelers bind actin and actin-related proteins (Arps) through an N-terminal helicase/SANT-associated (HSA) domain. Between the HSA and ATPase domains lies a conserved post-HSA (pHSA) domain. The HSA domain of Sth1, the catalytic subunit of the yeast SWI/SNF-family remodeler RSC, recruits the Rtt102-Arp7/9 heterotrimer. Rtt102-Arp7/9 regulates RSC function, but the mechanism is unclear. We show that the pHSA domain interacts directly with another conserved region of the catalytic subunit, protrusion-1. Rtt102-Arp7/9 binding to the HSA domain weakens this interaction and promotes the formation of stable, monodisperse complexes with DNA and nucleosomes. A crystal structure of Rtt102-Arp7/9 shows that ATP binds to Arp7 but not Arp9. However, Arp7 does not hydrolyze ATP. Together, the results suggest that Rtt102 and ATP stabilize a conformation of Arp7/9 that potentiates binding to the HSA domain, which releases intramolecular interactions within Sth1 and controls DNA and nucleosome binding.
Organizational Affiliation:
Department of Physiology, Perelman School of Medicine, University of Pennsylvania, 728 Clinical Research Building, 415 Curie Boulevard, Philadelphia, PA, 19104-6085, USA.