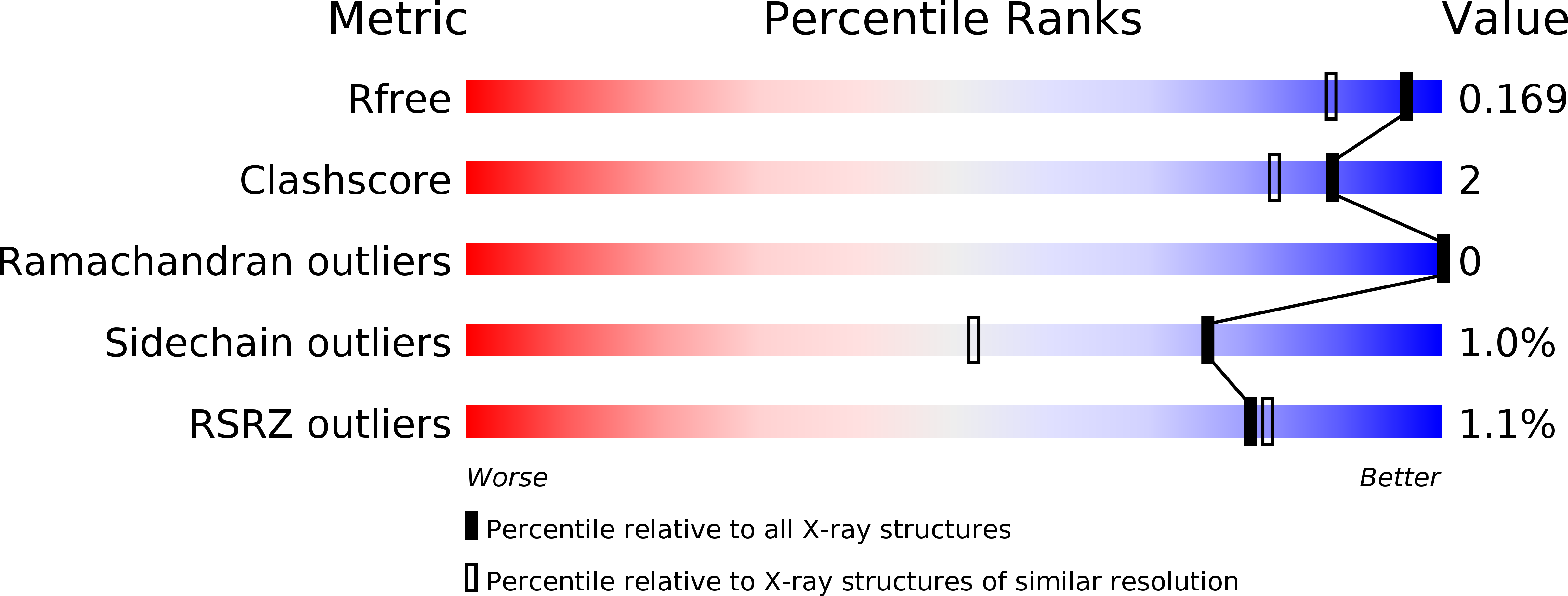
Molecular Basis of Substrate Recognition and Product Release by the Klebsiella pneumoniae Carbapenemase (KPC-2).
Pemberton, O.A., Zhang, X., Chen, Y.(2017) J Med Chem 60: 3525-3530
- PubMed: 28388065
- DOI: https://doi.org/10.1021/acs.jmedchem.7b00158
- Primary Citation of Related Structures:
5UJ3, 5UJ4, 5UL8 - PubMed Abstract:
Carbapenem-resistant Enterobacteriaceae are resistant to most β-lactam antibiotics due to the production of the Klebsiella pneumoniae carbapenemase (KPC-2) class A β-lactamase. Here, we present the first product complex crystal structures of KPC-2 with β-lactam antibiotics containing hydrolyzed cefotaxime and faropenem. They provide experimental insights into substrate recognition by KPC-2 and its unique cephalosporinase/carbapenemase activity. These structures also represent the first product complexes for a wild-type serine β-lactamase, elucidating the product release mechanism of these enzymes in general.
Organizational Affiliation:
Department of Molecular Medicine, University of South Florida , 12901 Bruce B. Downs Boulevard, Tampa, Florida 33612, United States.