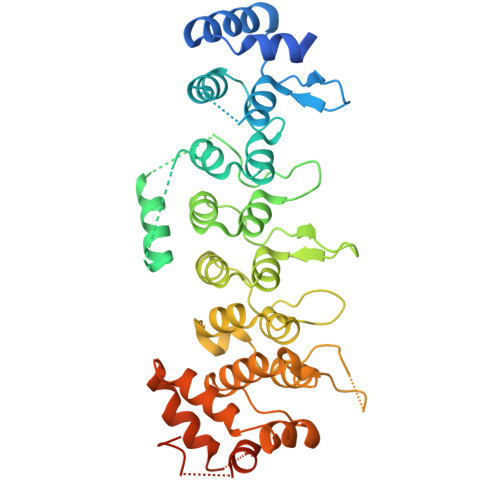
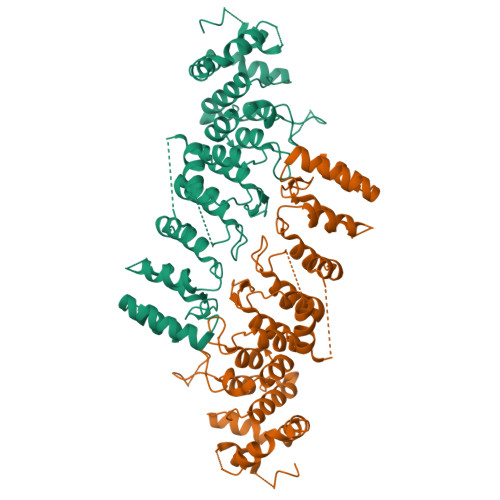
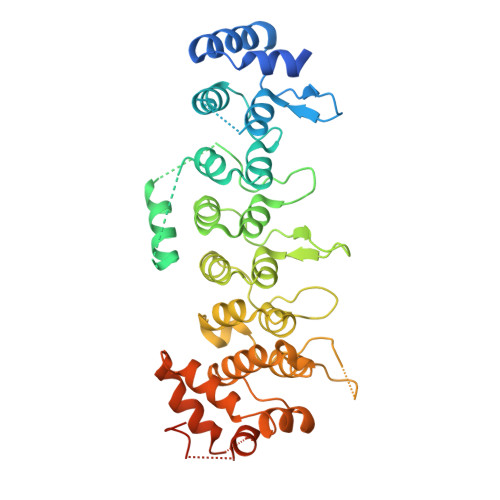
Ankyrin repeats as a dimerization module.
Kozlov, G., Wong, K., Wang, W., Skubak, P., Munoz-Escobar, J., Liu, Y., Siddiqui, N., Pannu, N.S., Gehring, K.(2018) Biochem Biophys Res Commun 495: 1002-1007
- PubMed: 29175332
- DOI: https://doi.org/10.1016/j.bbrc.2017.11.135
- Primary Citation of Related Structures:
5VRQ - PubMed Abstract:
Legionella pneumophila is a pathogen, causing severe pneumonia in humans called Legionnaires' disease. AnkC (LegA12) is a poorly characterized 495-residue effector protein conserved in multiple Legionella species. Here, we report the crystal structure of a C-terminally truncated AnkC (2-384) at 3.2 Å resolution. The structure shows seven ankyrin repeats (ARs) with unique structural features. AnkC forms a dimer along the outer surface of loops between ARs. The dimer exists both in the crystal form and in solution, as shown by analytical ultracentrifugation. This is the first example of ARs as a dimerization module as opposed to solely a protein interaction domain. In addition, a novel α-helix insert between AR3-AR4 is positioned across the surface opposite the ankyrin groove. Sequence conservation suggests that the ankyrin groove of AnkC is a functional site that interacts with binding targets. This ankyrin domain structure is an important step towards a functional characterization of AnkC.
Organizational Affiliation:
Department of Biochemistry, Groupe de recherche axé sur la structure des protéines, McGill University, Montreal, QC H3G 0B1, Canada.