Discovery of alpha-l-arabinopyranosidases from human gut microbiome expands the diversity within glycoside hydrolase family 42.
Viborg, A.H., Katayama, T., Arakawa, T., Abou Hachem, M., Lo Leggio, L., Kitaoka, M., Svensson, B., Fushinobu, S.(2017) J Biological Chem 292: 21092-21101
- PubMed: 29061847
- DOI: https://doi.org/10.1074/jbc.M117.792598
- Primary Citation of Related Structures:
5XB7 - PubMed Abstract:
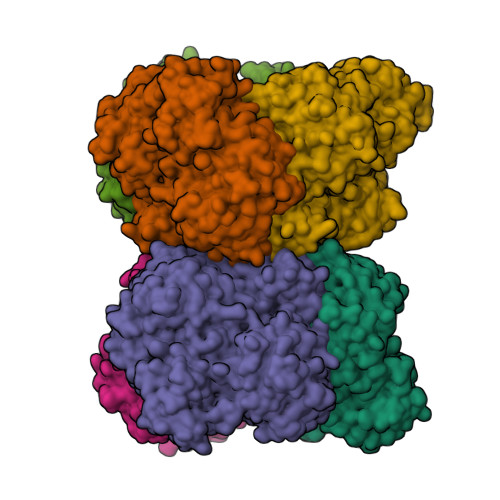
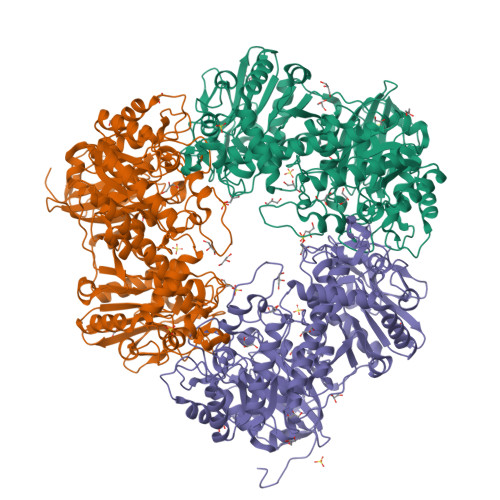
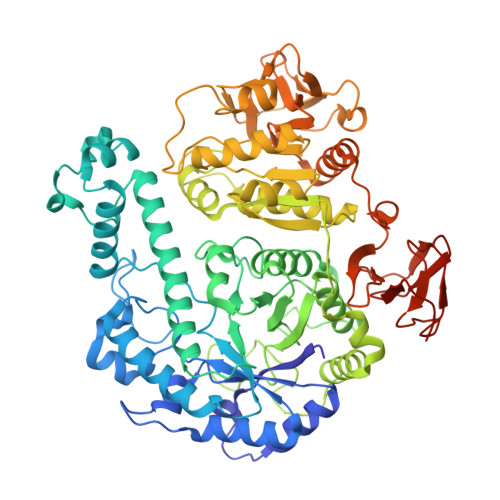
Enzymes of the glycoside hydrolase family 42 (GH42) are widespread in bacteria of the human gut microbiome and play fundamental roles in the decomposition of both milk and plant oligosaccharides. All GH42 enzymes characterized so far have β-galactosidase activity. Here, we report the existence of a GH42 subfamily that is exclusively specific for α-l-arabinopyranoside and describe the first representative of this subfamily. We found that this enzyme ( Bl Arap42B) from a probiotic Bifidobacterium species cannot hydrolyze β-galactosides. However, Bl Arap42B effectively hydrolyzed paeonolide and ginsenoside Rb2, plant glycosides containing an aromatic aglycone conjugated to α-l-arabinopyranosyl-(1,6)-β-d-glucopyranoside. Paeonolide, a natural glycoside from the roots of the plant genus Paeonia, is not hydrolyzed by classical GH42 β-galactosidases. X-ray crystallography revealed a unique Trp 345 - X 12 -Trp 358 sequence motif at the Bl Arap42B active site, as compared with a Phe- X 12 -His motif in classical GH42 β-galactosidases. This analysis also indicated that the C6 position of galactose is blocked by the aromatic side chains, hence allowing accommodation only of Ara p lacking this carbon. Automated docking of paeonolide revealed that it can fit into the Bl Ara p 42B active site. The Glc p moiety of paeonolide stacks onto the aromatic ring of the Trp 252 at subsite +1 and C4-OH is hydrogen bonded with Asp 249 Moreover, the aglycone stacks against Phe 421 from the neighboring monomer in the Bl Ara p 42B trimer, forming a proposed subsite +2. These results further support the notion that evolution of metabolic specialization can be tracked at the structural level in key enzymes facilitating degradation of specific glycans in an ecological niche.
Organizational Affiliation:
From the Department of Biotechnology, The University of Tokyo, Tokyo 113-8657, Japan.