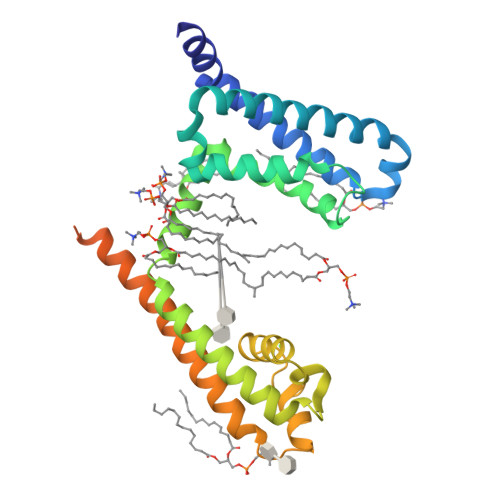
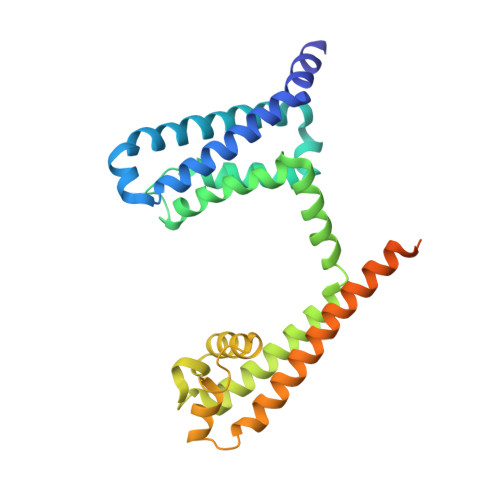
Structural insight on the voltage dependence of prokaryotic voltage gated sodium channel NavAb.
Irie, K., Haga, Y., Shimomura, T., Fujiyoshi, Y.(2017) FEBS Lett
- PubMed: 29274127
- DOI: https://doi.org/10.1002/1873-3468.12955
- Primary Citation of Related Structures:
5YUA, 5YUB, 5YUC - PubMed Abstract:
Voltage-gated sodium channels are crucial for electro-signalling in living systems. Analysis of the molecular mechanism requires both fine electrophysiological evaluation and high-resolution channel structures. Here, we optimized a dual expression system of NavAb, which is a well-established standard of prokaryotic voltage-gated sodium channels, for E. coli and insect cells using a single plasmid vector to analyse high-resolution protein structures and measure large ionic currents. Using this expression system, we evaluated the voltage dependence and determined the crystal structures of NavAb wild-type and two mutants, E32Q and N49K, whose voltage dependence were positively shifted and essential interactions were lost in voltage sensor domain. The structural and functional comparison elucidated the molecular mechanisms of the voltage dependence of prokaryotic voltage-gated sodium channels.
Organizational Affiliation:
Cellular and Structural Physiology Institute (CeSPI), Nagoya University, Japan.