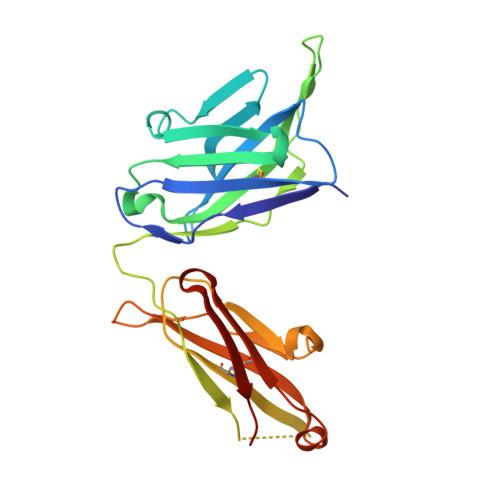
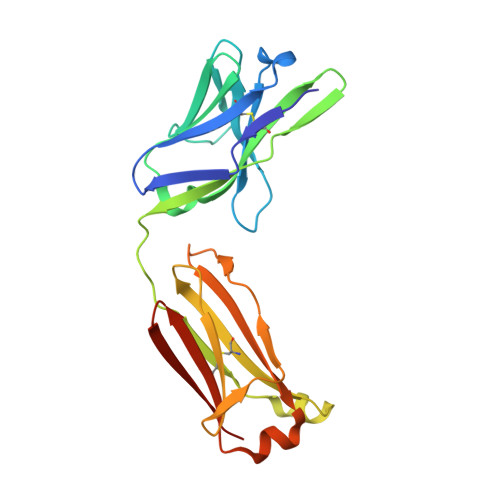
Structural basis for antibody targeting of the broadly expressed microbial polysaccharide poly-N-acetylglucosamine.
Soliman, C., Walduck, A.K., Yuriev, E., Richards, J.S., Cywes-Bentley, C., Pier, G.B., Ramsland, P.A.(2018) J Biol Chem 293: 5079-5089
- PubMed: 29449370
- DOI: https://doi.org/10.1074/jbc.RA117.001170
- Primary Citation of Related Structures:
6BE2, 6BE3, 6BE4 - PubMed Abstract:
In response to the widespread emergence of antibiotic-resistant microbes, new therapeutic agents are required for many human pathogens. A non-mammalian polysaccharide, poly- N -acetyl-d-glucosamine (PNAG), is produced by bacteria, fungi, and protozoan parasites. Antibodies that bind to PNAG and its deacetylated form (dPNAG) exhibit promising in vitro and in vivo activities against many microbes. A human IgG1 mAb (F598) that binds both PNAG and dPNAG has opsonic and protective activities against multiple microbial pathogens and is undergoing preclinical and clinical assessments as a broad-spectrum antimicrobial therapy. Here, to understand how F598 targets PNAG, we determined crystal structures of the unliganded F598 antigen-binding fragment (Fab) and its complexes with N -acetyl-d-glucosamine (GlcNAc) and a PNAG oligosaccharide. We found that F598 recognizes PNAG through a large groove-shaped binding site that traverses the entire light- and heavy-chain interface and accommodates at least five GlcNAc residues. The Fab-GlcNAc complex revealed a deep binding pocket in which the monosaccharide and a core GlcNAc of the oligosaccharide were almost identically positioned, suggesting an anchored binding mechanism of PNAG by F598. The Fab used in our structural analyses retained binding to PNAG on the surface of an antibiotic-resistant, biofilm-forming strain of Staphylococcus aureus Additionally, a model of intact F598 binding to two pentasaccharide epitopes indicates that the Fab arms can span at least 40 GlcNAc residues on an extended PNAG chain. Our findings unravel the structural basis for F598 binding to PNAG on microbial surfaces and biofilms.
Organizational Affiliation:
From the School of Science, Royal Melbourne Institute of Technology (RMIT) University, Bundoora, Victoria 3083, Australia.