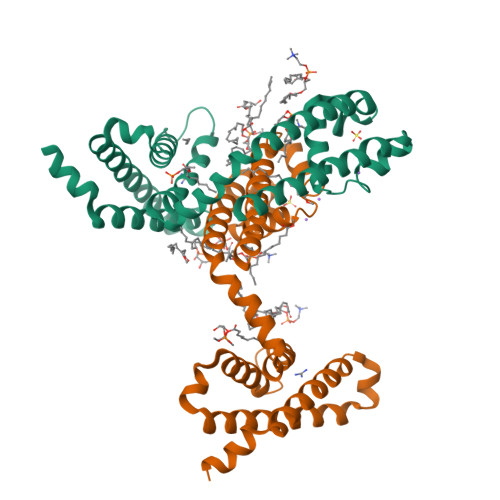
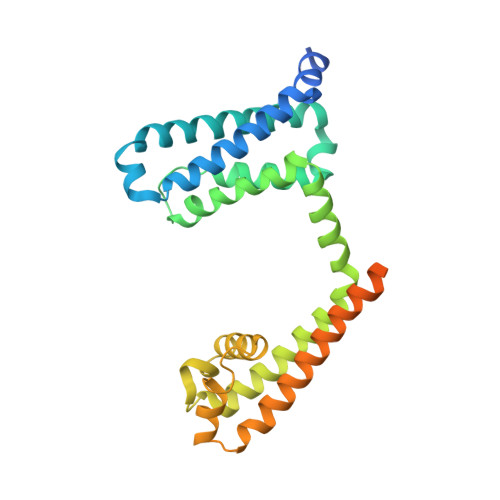
Structural basis for gating pore current in periodic paralysis.
Jiang, D., Gamal El-Din, T.M., Ing, C., Lu, P., Pomes, R., Zheng, N., Catterall, W.A.(2018) Nature 557: 590-594
- PubMed: 29769724
- DOI: https://doi.org/10.1038/s41586-018-0120-4
- Primary Citation of Related Structures:
6C1E, 6C1K, 6C1M, 6C1P - PubMed Abstract:
Potassium-sensitive hypokalaemic and normokalaemic periodic paralysis are inherited skeletal muscle diseases characterized by episodes of flaccid muscle weakness 1,2 . They are caused by single mutations in positively charged residues ('gating charges') in the S4 transmembrane segment of the voltage sensor of the voltage-gated sodium channel Na v 1.4 or the calcium channel Ca v 1.1 1,2 . Mutations of the outermost gating charges (R1 and R2) cause hypokalaemic periodic paralysis 1,2 by creating a pathogenic gating pore in the voltage sensor through which cations leak in the resting state 3,4 . Mutations of the third gating charge (R3) cause normokalaemic periodic paralysis 5 owing to cation leak in both activated and inactivated states 6 . Here we present high-resolution structures of the model bacterial sodium channel Na v Ab with the analogous gating-charge mutations 7,8 , which have similar functional effects as in the human channels. The R2G and R3G mutations have no effect on the backbone structures of the voltage sensor, but they create an aqueous cavity near the hydrophobic constriction site that controls gating charge movement through the voltage sensor. The R3G mutation extends the extracellular aqueous cleft through the entire length of the activated voltage sensor, creating an aqueous path through the membrane. Conversely, molecular modelling shows that the R2G mutation creates a continuous aqueous path through the membrane only in the resting state. Crystal structures of Na v Ab(R2G) in complex with guanidinium define a potential drug target site. Molecular dynamics simulations illustrate the mechanism of Na + permeation through the mutant gating pore in concert with conformational fluctuations of the gating charge R4. Our results reveal pathogenic mechanisms of periodic paralysis at the atomic level and suggest designs of drugs that may prevent ionic leak and provide symptomatic relief from hypokalaemic and normokalaemic periodic paralysis.
Organizational Affiliation:
Department of Pharmacology, University of Washington, Seattle, WA, USA.