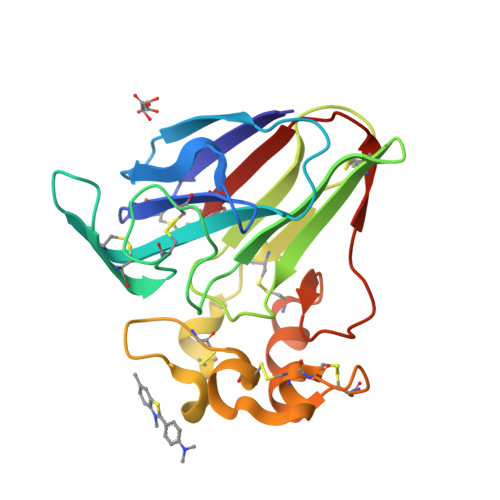
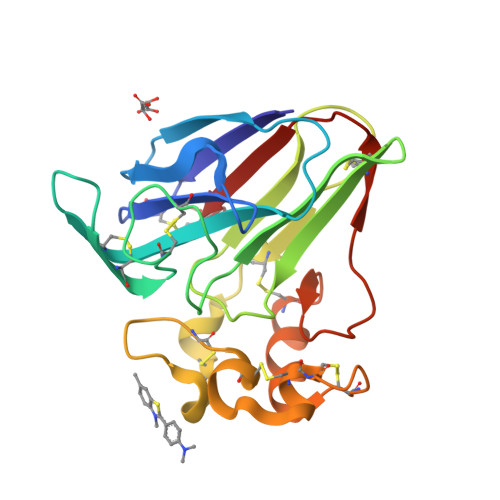
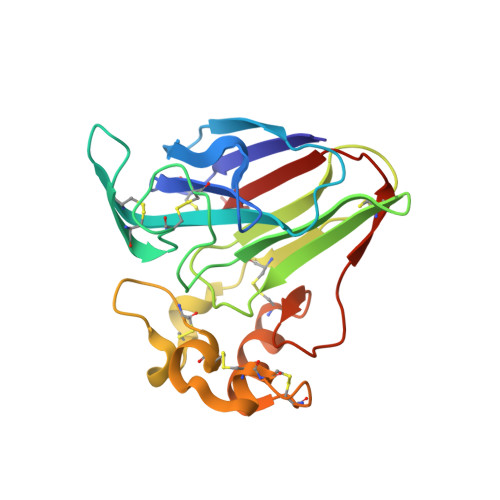
Investigation into the binding of dyes within protein crystals.
McPherson, A., Larson, S.B.(2018) Acta Crystallogr F Struct Biol Commun 74: 593-602
- PubMed: 30198893
- DOI: https://doi.org/10.1107/S2053230X18010300
- Primary Citation of Related Structures:
6C6W, 6E0D - PubMed Abstract:
It was found that the crystals of at least a dozen different proteins could be thoroughly stained to an intense color with a panel of dyes. Many, if not most, of the stained protein crystals retained the dyes almost indefinitely when placed in large volumes of dye-free mother liquor. Dialysis experiments showed that most of the dyes that were retained in crystals also bound to the protein when free in solution; less frequently, some dyes bound only in the crystal. The experiments indicated a strong association of the dyes with the proteins. Four protein crystals were investigated by X-ray diffraction to ascertain the mode of binding. These were crystals of lysozyme, thaumatin, trypsin inhibited with benzamidine and satellite tobacco mosaic virus. In 30 X-ray analyses of protein crystal-dye complexes, in only three difference Fourier maps was any difference electron density present that was consistent with the binding of dye molecules, and even in these three cases (thaumatin plus thioflavin T, xylene cyanol and m-cresol purple) the amount of dye observed was inadequate to explain the intense color of the crystals. It was concluded that the dye molecules, which are clearly inside the crystals, are disordered but are paradoxically tightly bound to the protein. It is speculated that the dyes, which exhibit large hydrophobic cores and peripheral charged groups, may interact with the crystalline proteins in the manner of conventional detergents.
Organizational Affiliation:
Molecular Biology and Biochemistry, University of California Irvine, 560 Steinhaus Hall, Irvine, CA 92697-3900, USA.