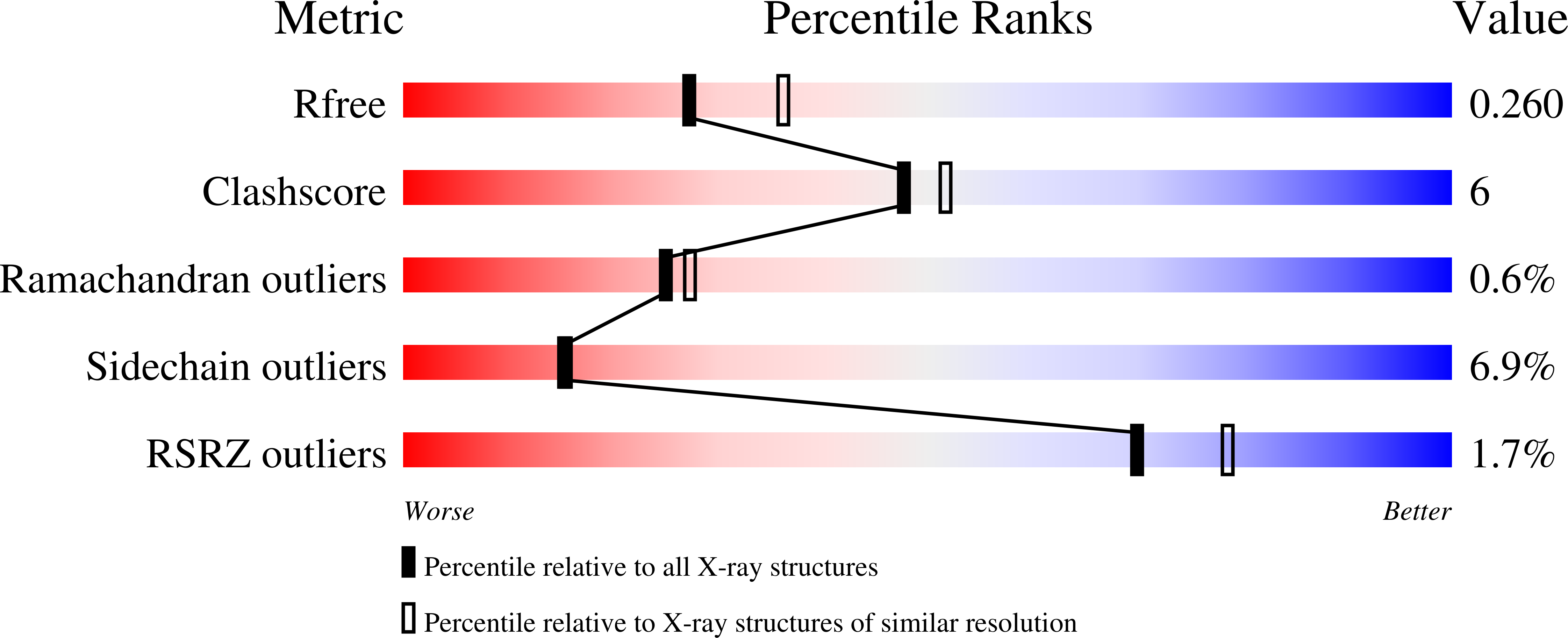
Structure-Activity Relationships in Metal-Binding Pharmacophores for Influenza Endonuclease.
Credille, C.V., Dick, B.L., Morrison, C.N., Stokes, R.W., Adamek, R.N., Wu, N.C., Wilson, I.A., Cohen, S.M.(2018) J Med Chem 61: 10206-10217
- PubMed: 30351002
- DOI: https://doi.org/10.1021/acs.jmedchem.8b01363
- Primary Citation of Related Structures:
6DCY, 6DCZ, 6DZQ, 6E0Q - PubMed Abstract:
Metalloenzymes represent an important target space for drug discovery. A limitation to the early development of metalloenzyme inhibitors has been the lack of established structure-activity relationships (SARs) for molecules that bind the metal ion cofactor(s) of a metalloenzyme. Herein, we employed a bioinorganic perspective to develop an SAR for inhibition of the metalloenzyme influenza RNA polymerase PA N endonuclease. The identified trends highlight the importance of the electronics of the metal-binding pharmacophore (MBP), in addition to MBP sterics, for achieving improved inhibition and selectivity. By optimization of the MBPs for PA N endonuclease, a class of highly active and selective fragments was developed that displays IC 50 values <50 nM. This SAR led to structurally distinct molecules that also displayed IC 50 values of ∼10 nM, illustrating the utility of a metal-centric development campaign in generating highly active and selective metalloenzyme inhibitors.
Organizational Affiliation:
Department of Chemistry and Biochemistry , University of California, San Diego , La Jolla , California 92093 , United States.