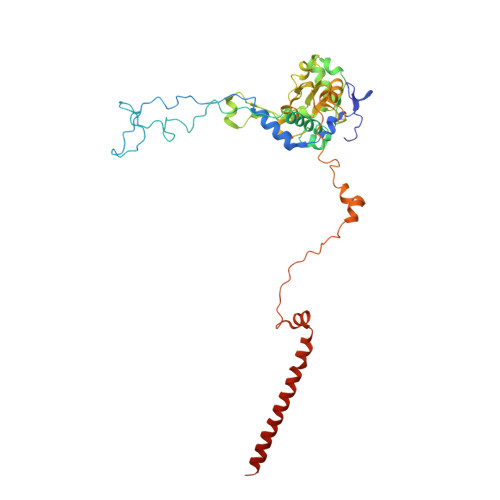
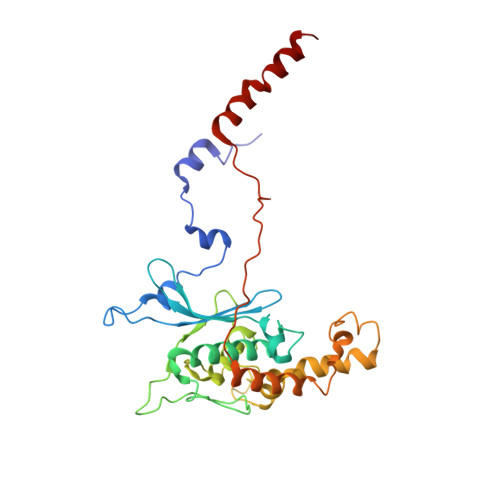
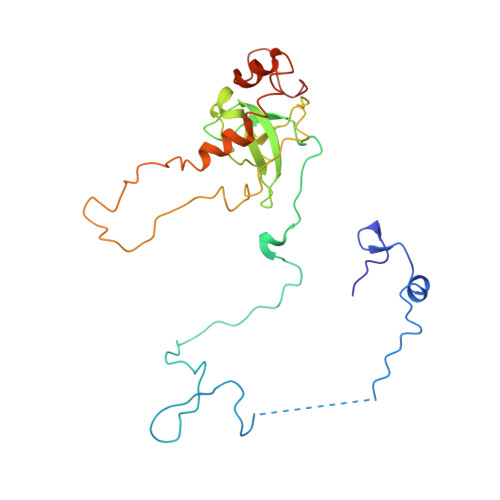
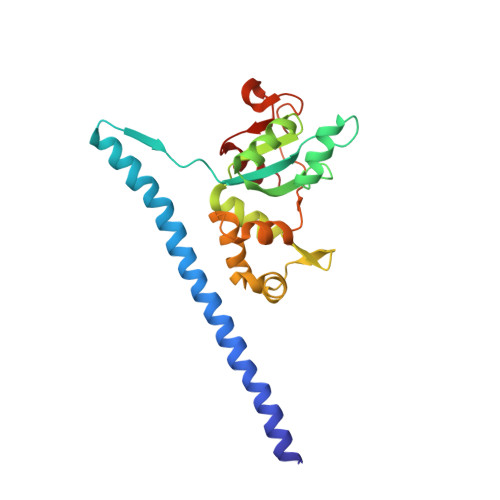
Structure of a prehandover mammalian ribosomal SRP·SRP receptor targeting complex.
Kobayashi, K., Jomaa, A., Lee, J.H., Chandrasekar, S., Boehringer, D., Shan, S.O., Ban, N.(2018) Science 360: 323-327
- PubMed: 29567807
- DOI: https://doi.org/10.1126/science.aar7924
- Primary Citation of Related Structures:
6FRK - PubMed Abstract:
Signal recognition particle (SRP) targets proteins to the endoplasmic reticulum (ER). SRP recognizes the ribosome synthesizing a signal sequence and delivers it to the SRP receptor (SR) on the ER membrane followed by the transfer of the signal sequence to the translocon. Here, we present the cryo-electron microscopy structure of the mammalian translating ribosome in complex with SRP and SR in a conformation preceding signal sequence handover. The structure visualizes all eukaryotic-specific SRP and SR proteins and reveals their roles in stabilizing this conformation by forming a large protein assembly at the distal site of SRP RNA. We provide biochemical evidence that the guanosine triphosphate hydrolysis of SRP·SR is delayed at this stage, possibly to provide a time window for signal sequence handover to the translocon.
Organizational Affiliation:
Department of Biology, Institute of Molecular Biology and Biophysics, ETH Zurich, Otto-Stern-Weg 5, Zurich CH-8093, Switzerland.