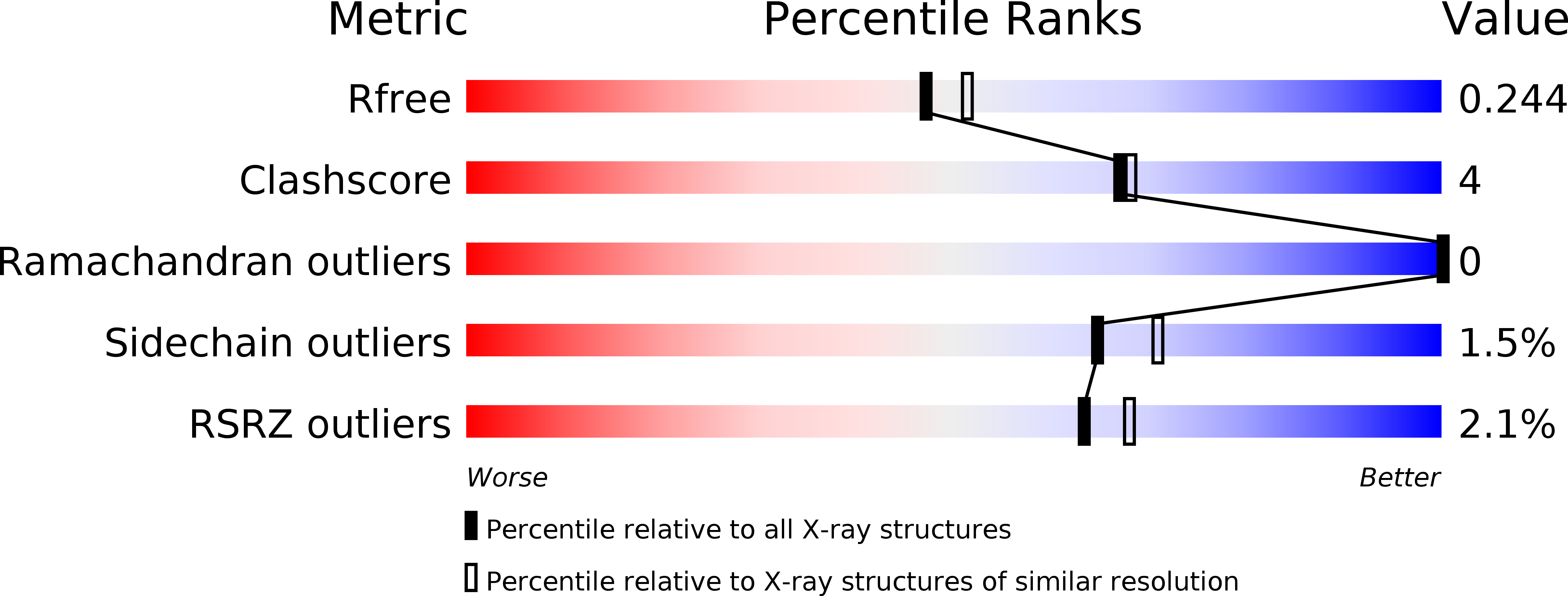
Makes caterpillars floppy-like effector-containing MARTX toxins require host ADP-ribosylation factor (ARF) proteins for systemic pathogenicity.
Lee, Y., Kim, B.S., Choi, S., Lee, E.Y., Park, S., Hwang, J., Kwon, Y., Hyun, J., Lee, C., Kim, J.F., Eom, S.H., Kim, M.H.(2019) Proc Natl Acad Sci U S A 116: 18031-18040
- PubMed: 31427506
- DOI: https://doi.org/10.1073/pnas.1905095116
- Primary Citation of Related Structures:
6II0, 6II2, 6II6, 6IMP - PubMed Abstract:
Upon invading target cells, multifunctional autoprocessing repeats-in-toxin (MARTX) toxins secreted by bacterial pathogens release their disease-related modularly structured effector domains. However, it is unclear how a diverse repertoire of effector domains within these toxins are processed and activated. Here, we report that Makes caterpillars floppy-like effector (MCF)-containing MARTX toxins require ubiquitous ADP-ribosylation factor (ARF) proteins for processing and activation of intermediate effector modules, which localize in different subcellular compartments following limited processing of holo effector modules by the internal cysteine protease. Effector domains structured tandemly with MCF in intermediate modules become disengaged and fully activated by MCF, which aggressively interacts with ARF proteins present at the same location as intermediate modules and is converted allosterically into a catalytically competent protease. MCF-mediated effector processing leads ultimately to severe virulence in mice via an MCF-mediated ARF switching mechanism across subcellular compartments. This work provides insight into how bacteria take advantage of host systems to induce systemic pathogenicity.
Organizational Affiliation:
Infection and Immunity Research Laboratory, Metabolic Regulation Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon 34141, Korea.