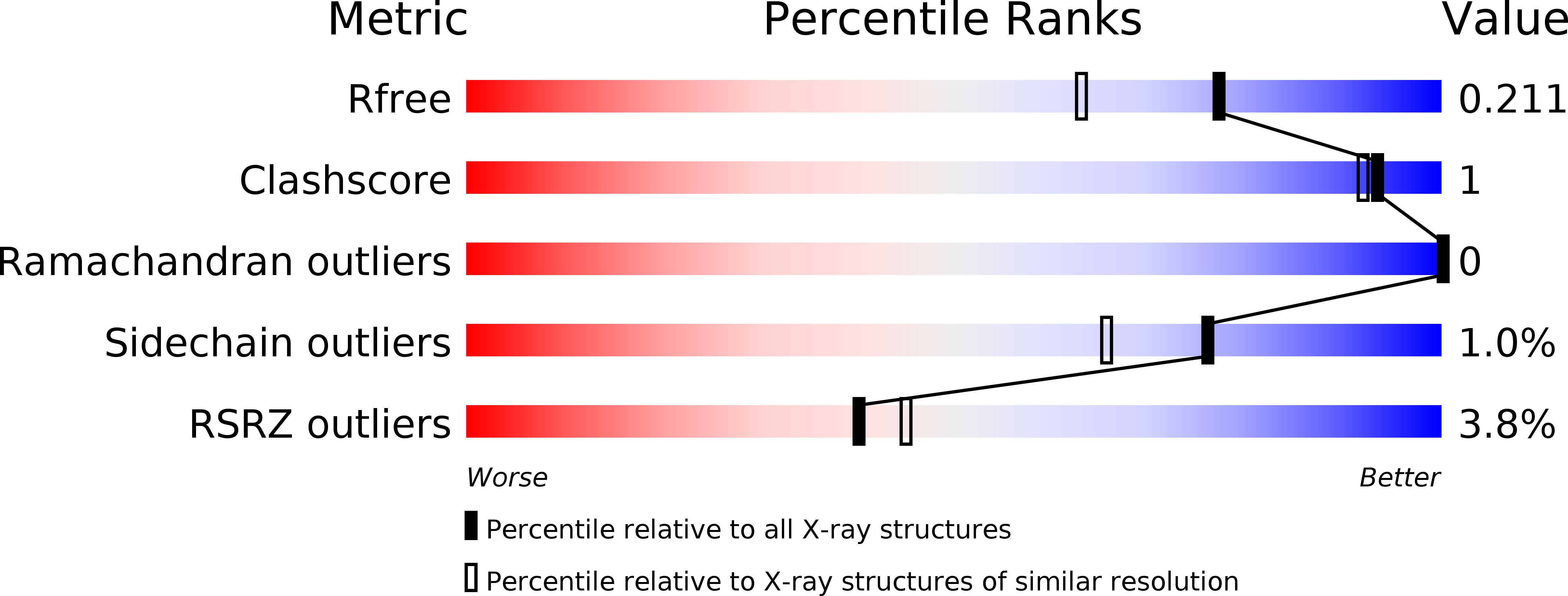
Structural and biochemical characterization of SADS-CoV papain-like protease 2.
Wang, L., Hu, W., Fan, C.(2020) Protein Sci 29: 1228-1241
- PubMed: 32216114
- DOI: https://doi.org/10.1002/pro.3857
- Primary Citation of Related Structures:
6L5T - PubMed Abstract:
Swine acute diarrhea syndrome coronavirus (SADS-CoV) is a novel coronavirus that is involved in severe diarrhea disease in piglets, causing considerable agricultural and economic loss in China. The emergence of this new coronavirus increases the importance of understanding SADS-CoV as well as antivirals. Coronaviral proteases, including main proteases and papain-like proteases (PLP), are attractive antiviral targets because of their essential roles in polyprotein processing and thus viral maturation. Here, we describe the biochemical and structural identification of recombinant SADS papain-like protease 2 (PLP2) domain of nsp3. The SADS-CoV PLP2 was shown to cleave nsp1 proteins and also peptides mimicking the nsp2|nsp3 cleavage site and also had deubiquitinating and deISGynating activity by in vitro assays. The crystal structure adopts an architecture resembling that of PLPs from other coronaviruses. We characterize both conserved and unique structural features likely directing the interaction of PLP2 with the substrates, including the tentative mapping of active site and other essential residues. These results provide a foundation for understanding the molecular basis of coronaviral PLPs' catalytic mechanism and for the screening and design of therapeutics to combat infection by SADS coronavirus.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Wuhan University, Wuhan, China.