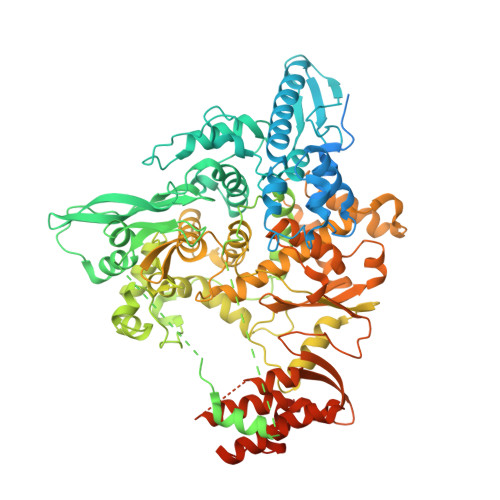
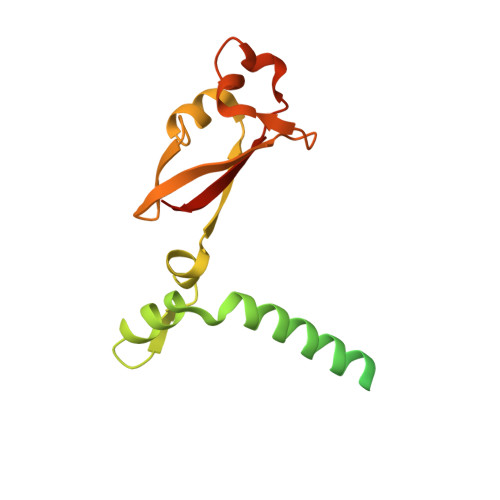
Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors.
Kirchdoerfer, R.N., Ward, A.B.(2019) Nat Commun 10: 2342-2342
- PubMed: 31138817
- DOI: https://doi.org/10.1038/s41467-019-10280-3
- Primary Citation of Related Structures:
6NUR, 6NUS - PubMed Abstract:
Recent history is punctuated by the emergence of highly pathogenic coronaviruses such as SARS- and MERS-CoV into human circulation. Upon infecting host cells, coronaviruses assemble a multi-subunit RNA-synthesis complex of viral non-structural proteins (nsp) responsible for the replication and transcription of the viral genome. Here, we present the 3.1 Å resolution structure of the SARS-CoV nsp12 polymerase bound to its essential co-factors, nsp7 and nsp8, using single particle cryo-electron microscopy. nsp12 possesses an architecture common to all viral polymerases as well as a large N-terminal extension containing a kinase-like fold and is bound by two nsp8 co-factors. This structure illuminates the assembly of the coronavirus core RNA-synthesis machinery, provides key insights into nsp12 polymerase catalysis and fidelity and acts as a template for the design of novel antiviral therapeutics.
Organizational Affiliation:
Department of Integrative Structural and Computational Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, HZ-102, La Jolla, CA, 92037, USA. rkirchdo@scripps.edu.