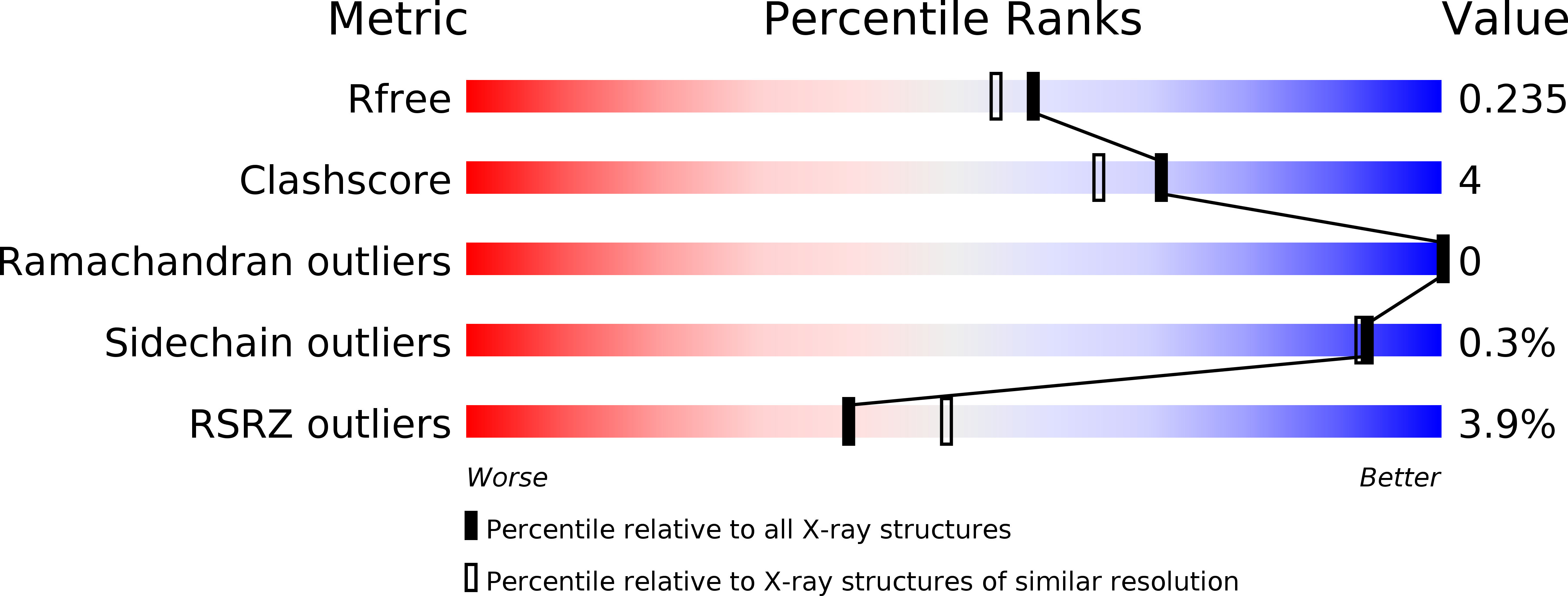
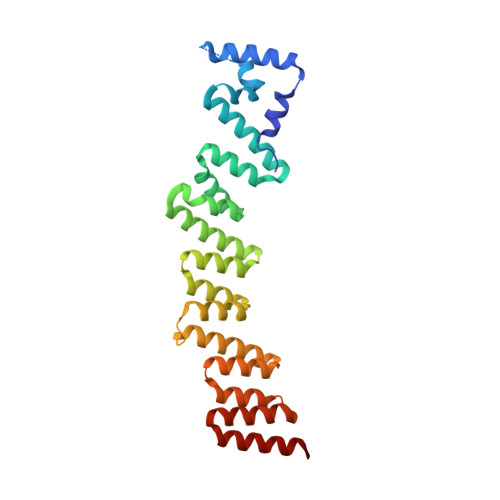
Structural basis for the dimerization of Gemin5 and its role in protein recruitment and translation control.
Moreno-Morcillo, M., Francisco-Velilla, R., Embarc-Buh, A., Fernandez-Chamorro, J., Ramon-Maiques, S., Martinez-Salas, E.(2020) Nucleic Acids Res 48: 788-801
- PubMed: 31799608
- DOI: https://doi.org/10.1093/nar/gkz1126
- Primary Citation of Related Structures:
6RNQ, 6RNS - PubMed Abstract:
In all organisms, a selected type of proteins accomplishes critical roles in cellular processes that govern gene expression. The multifunctional protein Gemin5 cooperates in translation control and ribosome binding, besides acting as the RNA-binding protein of the survival of motor neuron (SMN) complex. While these functions reside on distinct domains located at each end of the protein, the structure and function of the middle region remained unknown. Here, we solved the crystal structure of an extended tetratricopeptide (TPR)-like domain in human Gemin5 that self-assembles into a previously unknown canoe-shaped dimer. We further show that the dimerization module is functional in living cells driving the interaction between the viral-induced cleavage fragment p85 and the full-length Gemin5, which anchors splicing and translation members. Disruption of the dimerization surface by a point mutation in the TPR-like domain prevents this interaction and also abrogates translation enhancement induced by p85. The characterization of this unanticipated dimerization domain provides the structural basis for a role of the middle region of Gemin5 as a central hub for protein-protein interactions.
Organizational Affiliation:
Centro de Biología Molecular Severo Ochoa, CSIC-UAM, Nicolás Cabrera 1, 28049 Madrid, Spain.