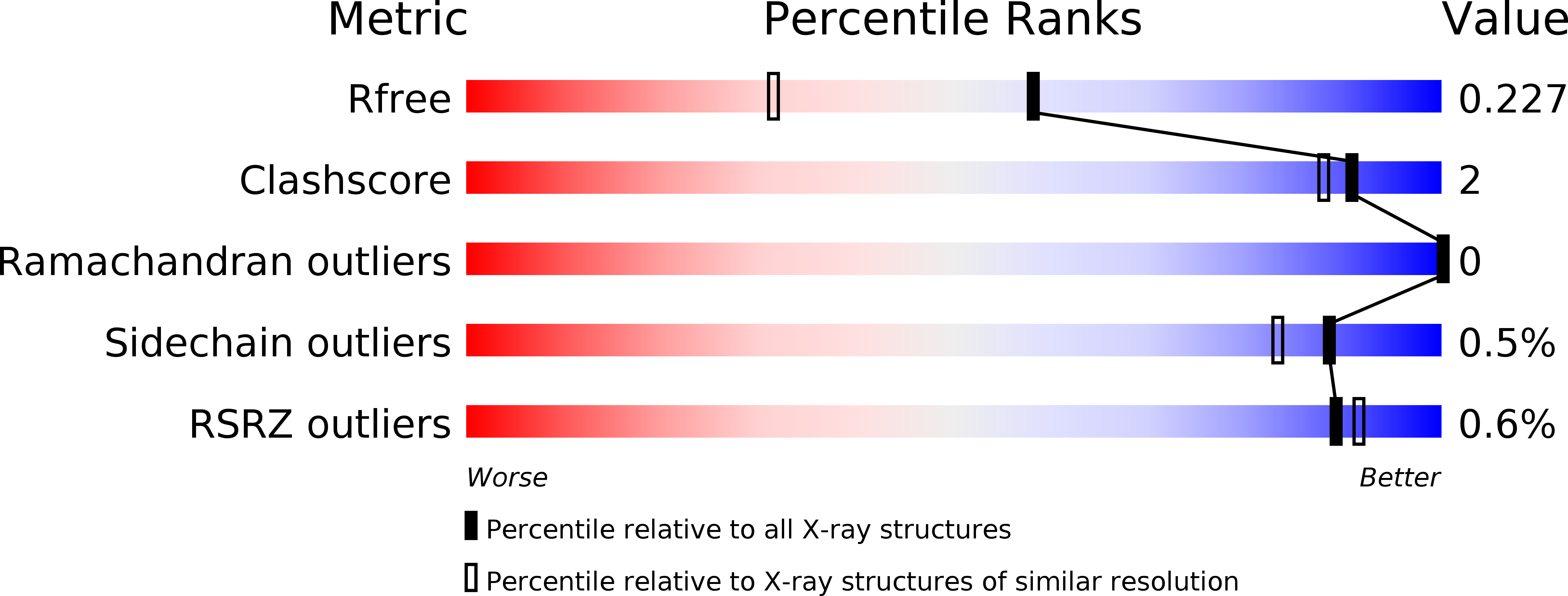
Substrate specificity of 2-deoxy-D-ribose 5-phosphate aldolase (DERA) assessed by different protein engineering and machine learning methods.
Voutilainen, S., Heinonen, M., Andberg, M., Jokinen, E., Maaheimo, H., Paakkonen, J., Hakulinen, N., Rouvinen, J., Lahdesmaki, H., Kaski, S., Rousu, J., Penttila, M., Koivula, A.(2020) Appl Microbiol Biotechnol 104: 10515-10529
- PubMed: 33147349
- DOI: https://doi.org/10.1007/s00253-020-10960-x
- Primary Citation of Related Structures:
6Z9H, 6Z9I, 6Z9J - PubMed Abstract:
In this work, deoxyribose-5-phosphate aldolase (Ec DERA, EC 4.1.2.4) from Escherichia coli was chosen as the protein engineering target for improving the substrate preference towards smaller, non-phosphorylated aldehyde donor substrates, in particular towards acetaldehyde. The initial broad set of mutations was directed to 24 amino acid positions in the active site or in the close vicinity, based on the 3D complex structure of the E. coli DERA wild-type aldolase. The specific activity of the DERA variants containing one to three amino acid mutations was characterised using three different substrates. A novel machine learning (ML) model utilising Gaussian processes and feature learning was applied for the 3rd mutagenesis round to predict new beneficial mutant combinations. This led to the most clear-cut (two- to threefold) improvement in acetaldehyde (C2) addition capability with the concomitant abolishment of the activity towards the natural donor molecule glyceraldehyde-3-phosphate (C3P) as well as the non-phosphorylated equivalent (C3). The Ec DERA variants were also tested on aldol reaction utilising formaldehyde (C1) as the donor. Ec DERA wild-type was shown to be able to carry out this reaction, and furthermore, some of the improved variants on acetaldehyde addition reaction turned out to have also improved activity on formaldehyde. KEY POINTS: • DERA aldolases are promiscuous enzymes. • Synthetic utility of DERA aldolase was improved by protein engineering approaches. • Machine learning methods aid the protein engineering of DERA.
Organizational Affiliation:
VTT Technical Research Centre of Finland Ltd, P.O. Box 1000, FI-02044 VTT, Espoo, Finland. sanni.voutilainen@vtt.fi.