The Bacterial [Fe]-Hydrogenase Paralog HmdII Uses Tetrahydrofolate Derivatives as Substrates.
Watanabe, T., Wagner, T., Huang, G., Kahnt, J., Ataka, K., Ermler, U., Shima, S.(2019) Angew Chem Int Ed Engl 58: 3506-3510
- PubMed: 30600878
- DOI: https://doi.org/10.1002/anie.201813465
- Primary Citation of Related Structures:
6HUX, 6HUY, 6HUZ - PubMed Abstract:
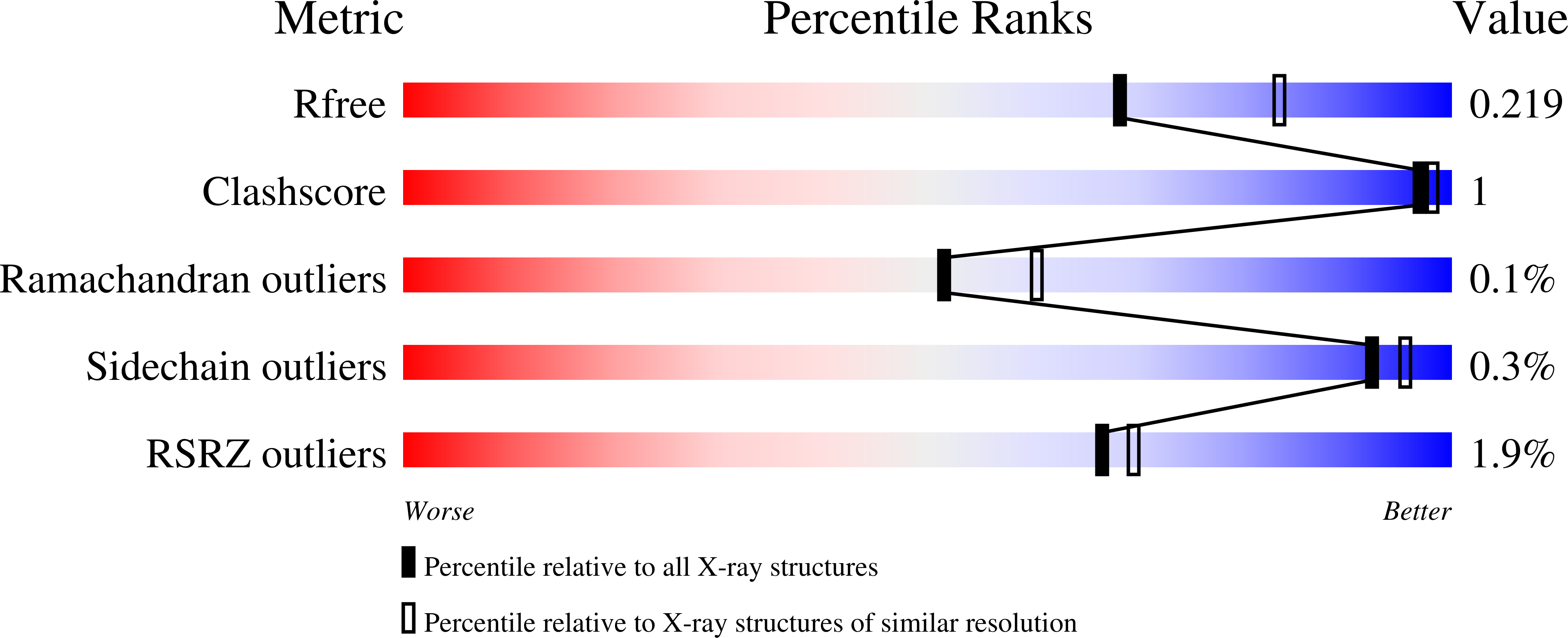
[Fe]-hydrogenase (Hmd) catalyzes the reversible hydrogenation of methenyl-tetrahydromethanopterin (methenyl-H 4 MPT + ) with H 2 . H 4 MPT is a C1-carrier of methanogenic archaea. One bacterial genus, Desulfurobacterium, contains putative genes for the Hmd paralog, termed HmdII, and the HcgA-G proteins. The latter are required for the biosynthesis of the prosthetic group of Hmd, the iron-guanylylpyridinol (FeGP) cofactor. This finding is intriguing because Hmd and HmdII strictly use H 4 MPT derivatives that are absent in most bacteria. We identified the presence of the FeGP cofactor in D. thermolithotrophum. The bacterial HmdII reconstituted with the FeGP cofactor catalyzed the hydrogenation of derivatives of tetrahydrofolate, the bacterial C1-carrier, albeit with low enzymatic activities. The crystal structures show how Hmd recognizes tetrahydrofolate derivatives. These findings have an impact on future biotechnology by identifying a bacterial Hmd paralog.
Organizational Affiliation:
Microbial Protein Structure Group, Max Planck Institute for Terrestrial Microbiology, Karl-von-Frisch Straße 10, 35043, Marburg, Germany.