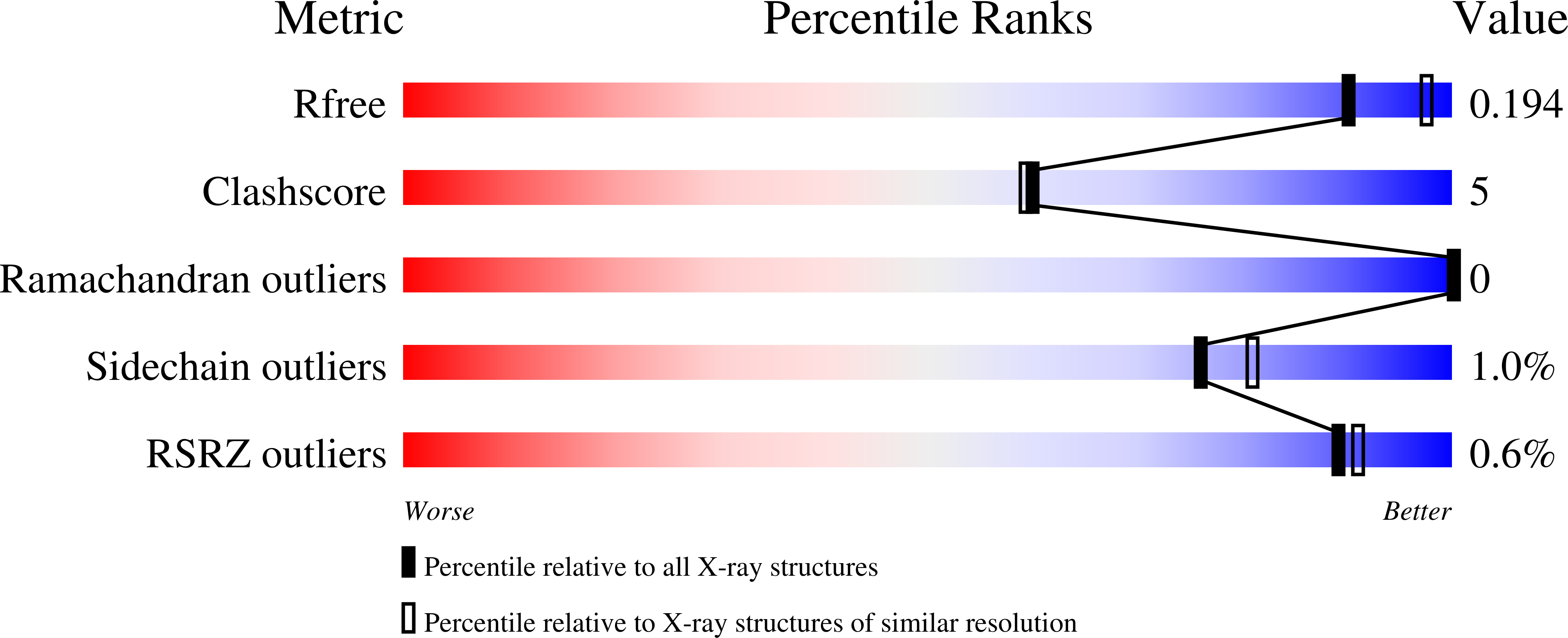
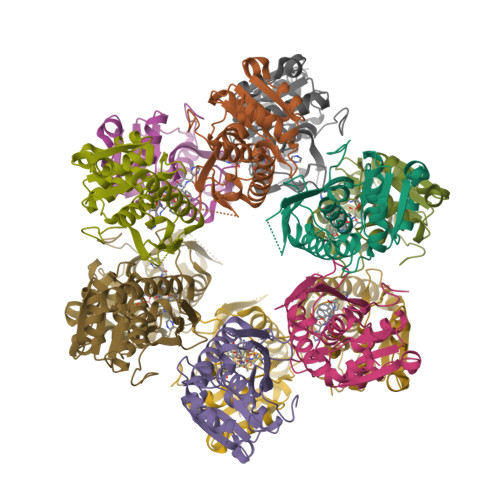
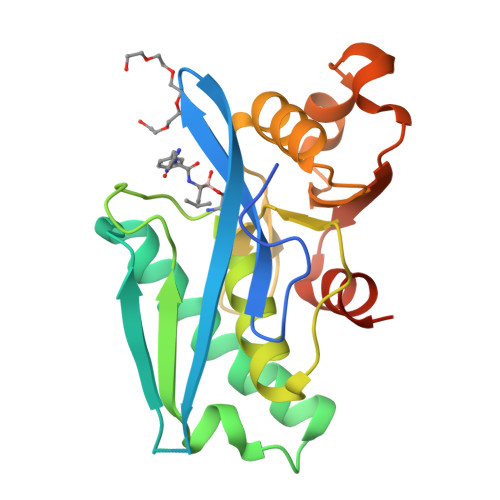
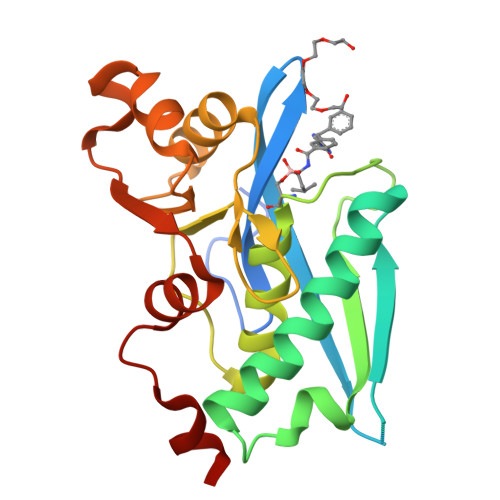
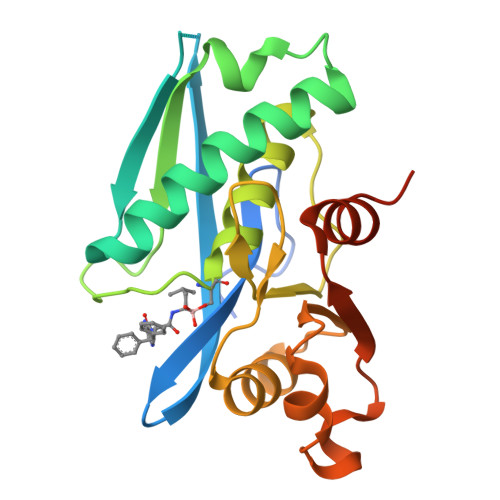
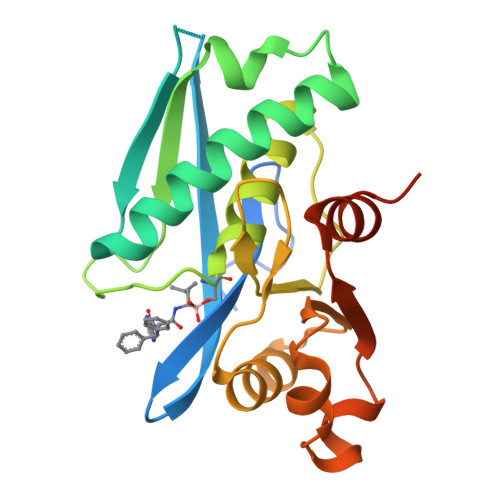
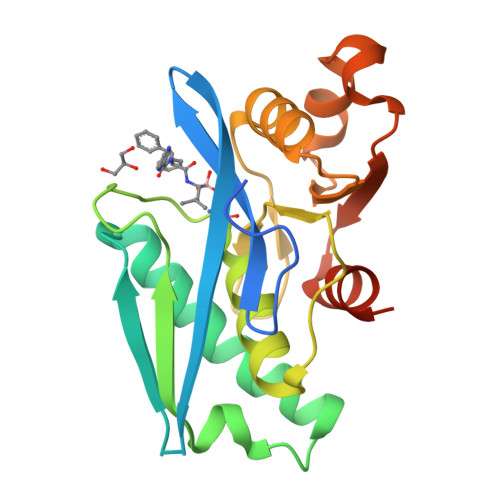
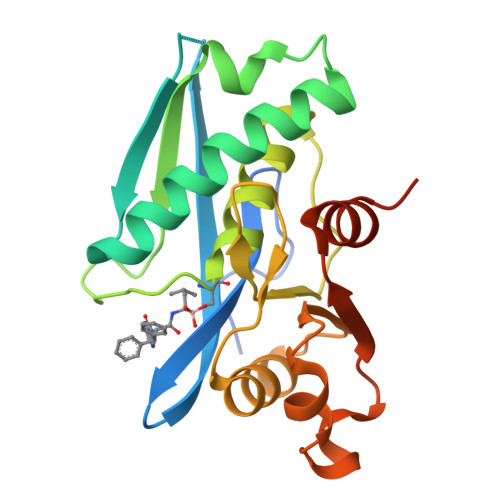
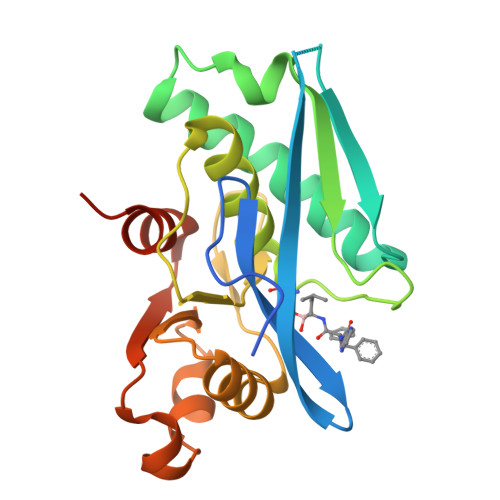
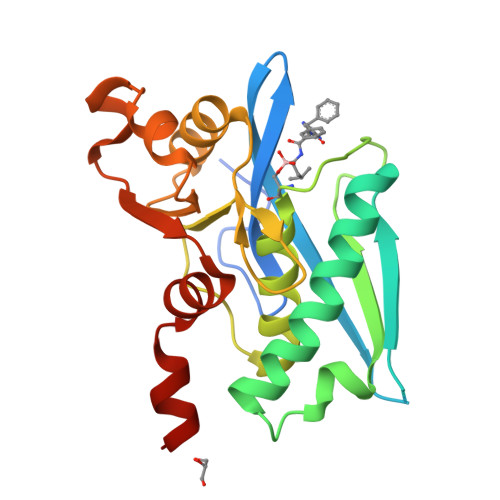
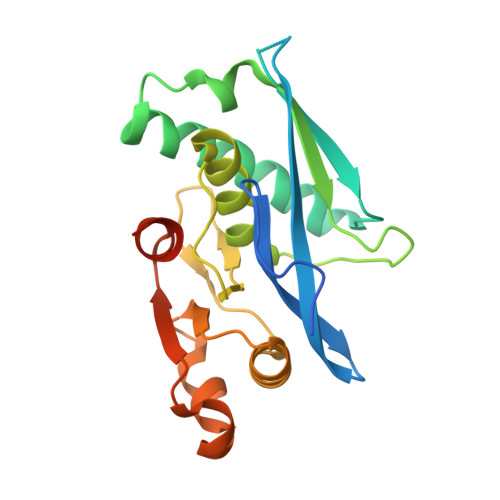
Structure-Based Design of Selective LONP1 Inhibitors for Probing In Vitro Biology.
Kingsley, L.J., He, X., McNeill, M., Nelson, J., Nikulin, V., Ma, Z., Lu, W., Zhou, V.W., Manuia, M., Kreusch, A., Gao, M.Y., Witmer, D., Vaillancourt, M.T., Lu, M., Greenblatt, S., Lee, C., Vashisht, A., Bender, S., Spraggon, G., Michellys, P.Y., Jia, Y., Haling, J.R., Lelais, G.(2021) J Med Chem 64: 4857-4869
- PubMed: 33821636
- DOI: https://doi.org/10.1021/acs.jmedchem.0c02152
- Primary Citation of Related Structures:
6WYS, 6WZV, 6X1M, 6X27 - PubMed Abstract:
LONP1 is an AAA+ protease that maintains mitochondrial homeostasis by removing damaged or misfolded proteins. Elevated activity and expression of LONP1 promotes cancer cell proliferation and resistance to apoptosis-inducing reagents. Despite the importance of LONP1 in human biology and disease, very few LONP1 inhibitors have been described in the literature. Herein, we report the development of selective boronic acid-based LONP1 inhibitors using structure-based drug design as well as the first structures of human LONP1 bound to various inhibitors. Our efforts led to several nanomolar LONP1 inhibitors with little to no activity against the 20S proteasome that serve as tool compounds to investigate LONP1 biology.
Organizational Affiliation:
Genomics Institute of the Novartis Research Foundation, 10675 John J. Hopkins Dr., San Diego, California 92121, United States.