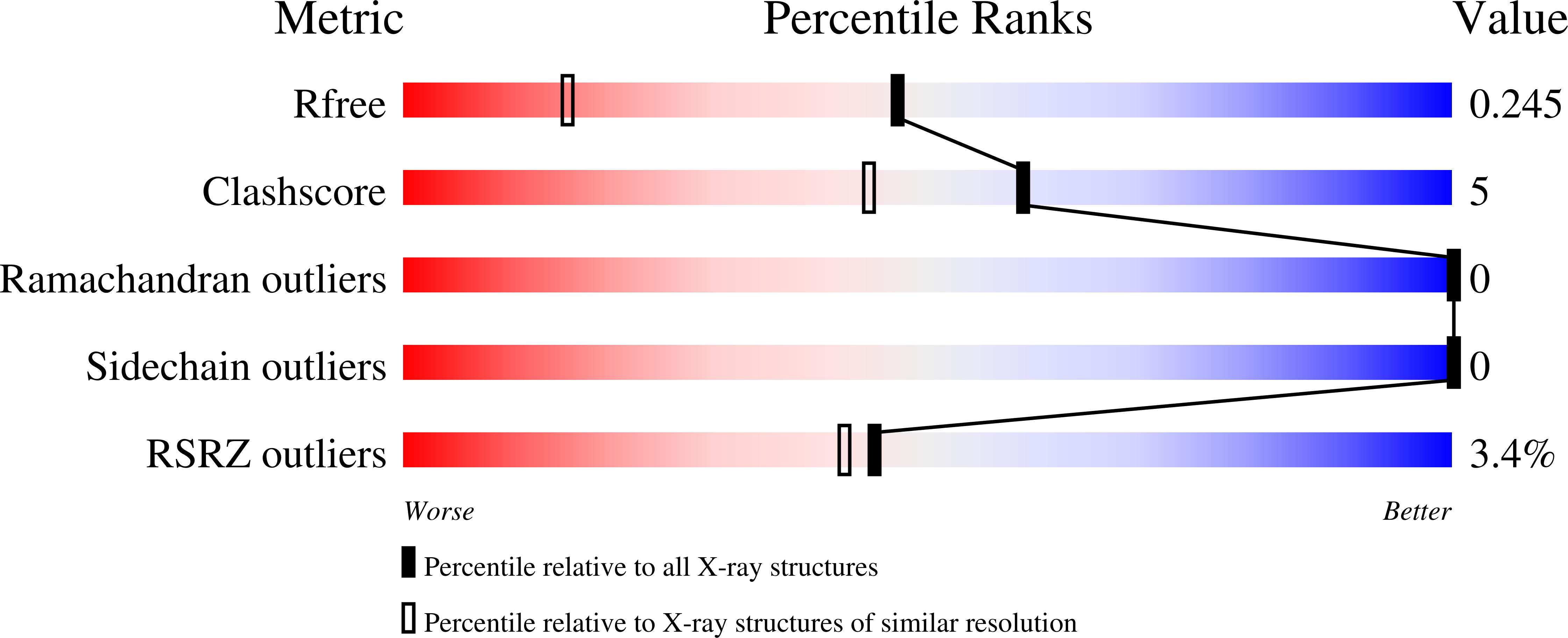
Structure-Function Dataset Reveals Environment Effects within a Fluorescent Protein Model System*.
De Zitter, E., Hugelier, S., Duwe, S., Vandenberg, W., Tebo, A.G., Van Meervelt, L., Dedecker, P.(2021) Angew Chem Int Ed Engl 60: 10073-10081
- PubMed: 33543524
- DOI: https://doi.org/10.1002/anie.202015201
- Primary Citation of Related Structures:
7A7K, 7A7L, 7A7M, 7A7N, 7A7O, 7A7P, 7A7Q, 7A7R, 7A7S, 7A7T, 7A7U, 7A7V, 7A7W, 7A7X, 7A7Y, 7A7Z, 7A80, 7A81, 7A82, 7A83, 7A84, 7A85, 7A86, 7A87, 7A88, 7A89, 7A8A, 7A8B, 7A8C, 7A8D, 7A8E, 7A8F, 7A8G, 7A8H, 7A8I, 7A8J, 7A8K, 7A8L, 7A8M, 7A8N, 7A8O - PubMed Abstract:
Anisotropic environments can drastically alter the spectroscopy and photochemistry of molecules, leading to complex structure-function relationships. We examined this using fluorescent proteins as easy-to-modify model systems. Starting from a single scaffold, we have developed a range of 27 photochromic fluorescent proteins that cover a broad range of spectroscopic properties, including the determination of 43 crystal structures. Correlation and principal component analysis confirmed the complex relationship between structure and spectroscopy, but also allowed us to identify consistent trends and to relate these to the spatial organization. We find that changes in spectroscopic properties can come about through multiple underlying mechanisms, of which polarity, hydrogen bonding and presence of water molecules are key modulators. We anticipate that our findings and rich structure/spectroscopy dataset can open opportunities for the development and evaluation of new and existing protein engineering methods.
Organizational Affiliation:
Department of Chemistry, KU Leuven, Celestijnenlaan 200G - box 2403, 3001, Leuven, Belgium.