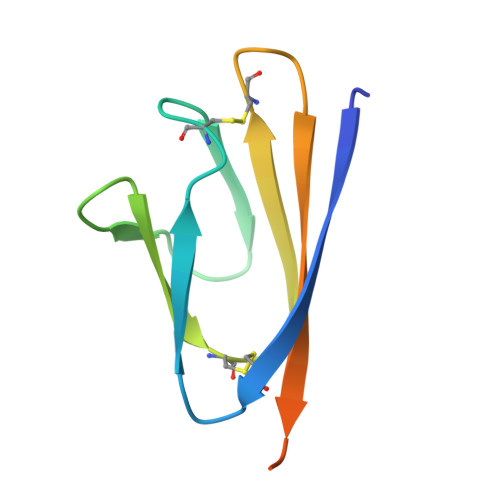
Structural insight reveals SARS-CoV-2 ORF7a as an immunomodulating factor for human CD14 + monocytes.
Zhou, Z., Huang, C., Zhou, Z., Huang, Z., Su, L., Kang, S., Chen, X., Chen, Q., He, S., Rong, X., Xiao, F., Chen, J., Chen, S.(2021) iScience 24: 102187-102187
- PubMed: 33615195
- DOI: https://doi.org/10.1016/j.isci.2021.102187
- Primary Citation of Related Structures:
7CI3 - PubMed Abstract:
Dysregulated immune cell responses have been linked to the severity of coronavirus disease 2019 (COVID-19), but the specific viral factors of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were currently unknown. Herein, we reveal that the Immunoglobulin-like fold ectodomain of the viral protein SARS-CoV-2 ORF7a interacts with high efficiency to CD14 + monocytes in human peripheral blood, compared to pathogenic protein SARS-CoV ORF7a. The crystal structure of SARS-CoV-2 ORF7a at 2.2 Å resolution reveals three remarkable changes on the amphipathic side of the four-stranded β-sheet, implying a potential functional interface of the viral protein. Importantly, SARS-CoV-2 ORF7a coincubation with CD14 + monocytes ex vivo triggered a decrease in HLA-DR/DP/DQ expression levels and upregulated significant production of proinflammatory cytokines, including IL-6, IL-1β, IL-8, and TNF-α. Our work demonstrates that SARS-CoV-2 ORF7a is an immunomodulating factor for immune cell binding and triggers dramatic inflammatory responses, providing promising therapeutic drug targets for pandemic COVID-19.
Organizational Affiliation:
Molecular Imaging Center, Guangdong Provincial Key Laboratory of Biomedical Imaging, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai 519000, China.