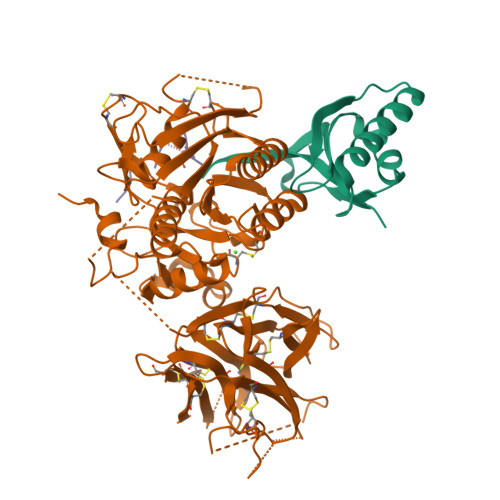
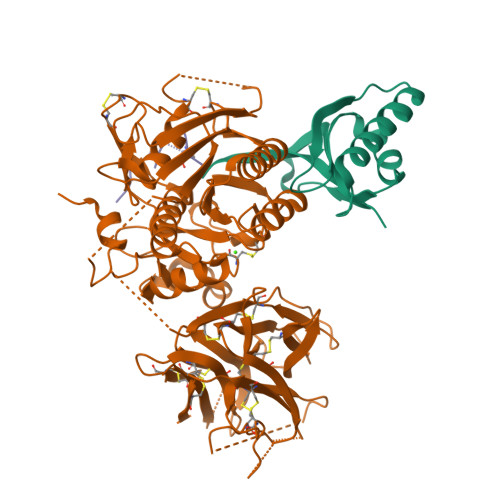
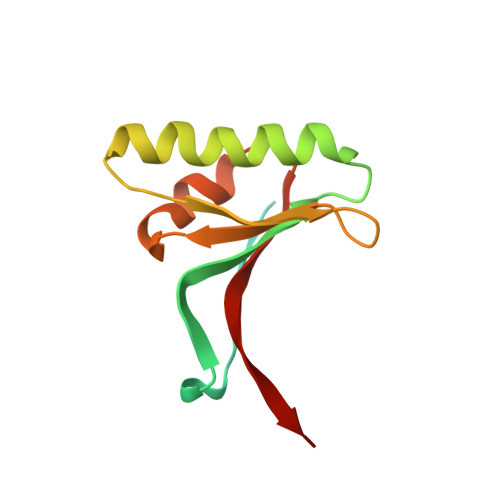
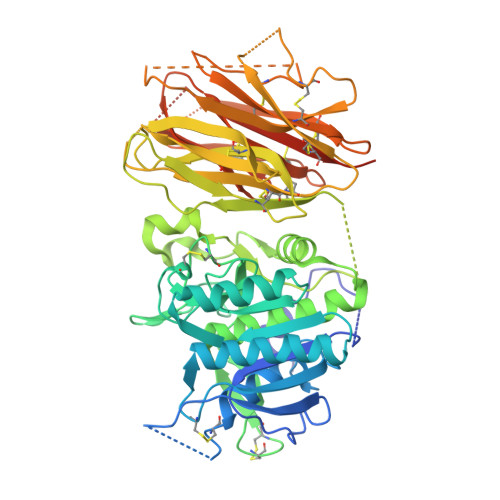
Identification of a PCSK9-LDLR disruptor peptide with in vivo function.
Brousseau, M.E., Clairmont, K.B., Spraggon, G., Flyer, A.N., Golosov, A.A., Grosche, P., Amin, J., Andre, J., Burdick, D., Caplan, S., Chen, G., Chopra, R., Ames, L., Dubiel, D., Fan, L., Gattlen, R., Kelly-Sullivan, D., Koch, A.W., Lewis, I., Li, J., Liu, E., Lubicka, D., Marzinzik, A., Nakajima, K., Nettleton, D., Ottl, J., Pan, M., Patel, T., Perry, L., Pickett, S., Poirier, J., Reid, P.C., Pelle, X., Seepersaud, M., Subramanian, V., Vera, V., Xu, M., Yang, L., Yang, Q., Yu, J., Zhu, G., Monovich, L.G.(2022) Cell Chem Biol 29: 249
- PubMed: 34547225
- DOI: https://doi.org/10.1016/j.chembiol.2021.08.012
- Primary Citation of Related Structures:
7KEV, 7KFA - PubMed Abstract:
Proprotein convertase subtilisin/kexin type 9 (PCSK9) regulates plasma low-density lipoprotein cholesterol (LDL-C) levels by promoting hepatic LDL receptor (LDLR) degradation. Therapeutic antibodies that disrupt PCSK9-LDLR binding reduce LDL-C concentrations and cardiovascular disease risk. The epidermal growth factor precursor homology domain A (EGF-A) of the LDLR serves as a primary contact with PCSK9 via a flat interface, presenting a challenge for identifying small molecule PCSK9-LDLR disruptors. We employ an affinity-based screen of 10 13 in vitro-translated macrocyclic peptides to identify high-affinity PCSK9 ligands that utilize a unique, induced-fit pocket and partially disrupt the PCSK9-LDLR interaction. Structure-based design led to molecules with enhanced function and pharmacokinetic properties (e.g., 13 PCSK9i). In mice, 13 PCSK9i reduces plasma cholesterol levels and increases hepatic LDLR density in a dose-dependent manner. 13 PCSK9i functions by a unique, allosteric mechanism and is the smallest molecule identified to date with in vivo PCSK9-LDLR disruptor function.
Organizational Affiliation:
Novartis Institutes for BioMedical Research, 22 Windsor Street and 181 Massachusetts Avenue, Cambridge, MA 02139, USA. Electronic address: margaret.brousseau@novartis.com.