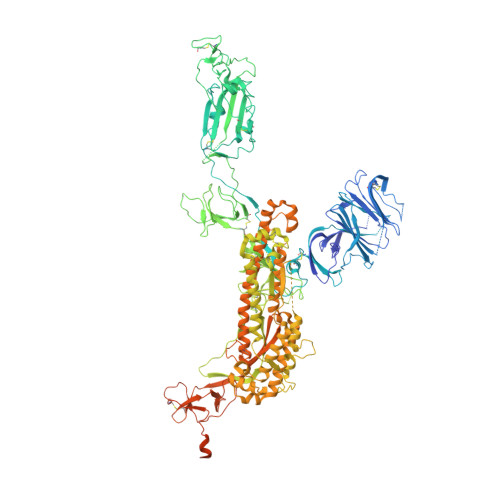
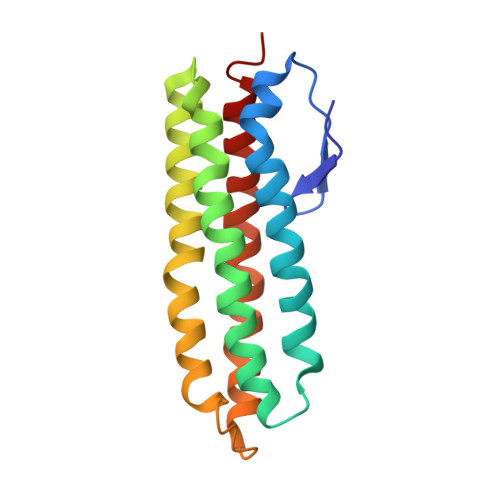
De novo design of potent and resilient hACE2 decoys to neutralize SARS-CoV-2.
Linsky, T.W., Vergara, R., Codina, N., Nelson, J.W., Walker, M.J., Su, W., Barnes, C.O., Hsiang, T.Y., Esser-Nobis, K., Yu, K., Reneer, Z.B., Hou, Y.J., Priya, T., Mitsumoto, M., Pong, A., Lau, U.Y., Mason, M.L., Chen, J., Chen, A., Berrocal, T., Peng, H., Clairmont, N.S., Castellanos, J., Lin, Y.R., Josephson-Day, A., Baric, R.S., Fuller, D.H., Walkey, C.D., Ross, T.M., Swanson, R., Bjorkman, P.J., Gale Jr., M., Blancas-Mejia, L.M., Yen, H.L., Silva, D.A.(2020) Science 370: 1208-1214
- PubMed: 33154107
- DOI: https://doi.org/10.1126/science.abe0075
- Primary Citation of Related Structures:
7KL9 - PubMed Abstract:
We developed a de novo protein design strategy to swiftly engineer decoys for neutralizing pathogens that exploit extracellular host proteins to infect the cell. Our pipeline allowed the design, validation, and optimization of de novo human angiotensin-converting enzyme 2 (hACE2) decoys to neutralize severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The best monovalent decoy, CTC-445.2, bound with low nanomolar affinity and high specificity to the receptor-binding domain (RBD) of the spike protein. Cryo-electron microscopy (cryo-EM) showed that the design is accurate and can simultaneously bind to all three RBDs of a single spike protein. Because the decoy replicates the spike protein target interface in hACE2, it is intrinsically resilient to viral mutational escape. A bivalent decoy, CTC-445.2d, showed ~10-fold improvement in binding. CTC-445.2d potently neutralized SARS-CoV-2 infection of cells in vitro, and a single intranasal prophylactic dose of decoy protected Syrian hamsters from a subsequent lethal SARS-CoV-2 challenge.
Organizational Affiliation:
Neoleukin Therapeutics Inc., Seattle, WA, USA.