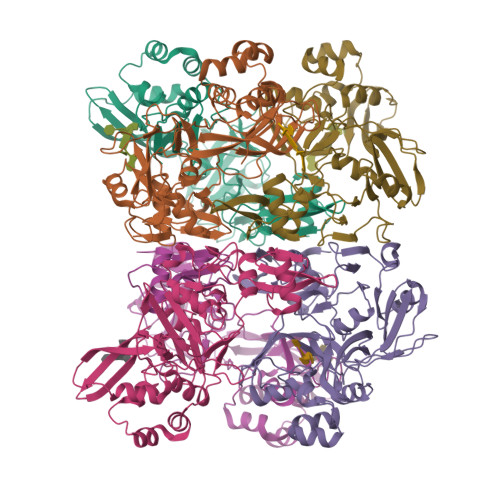
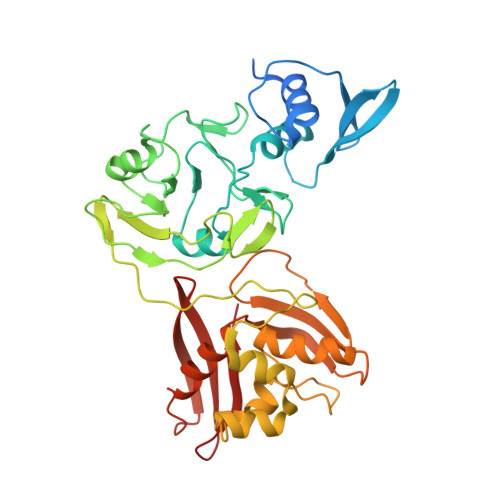
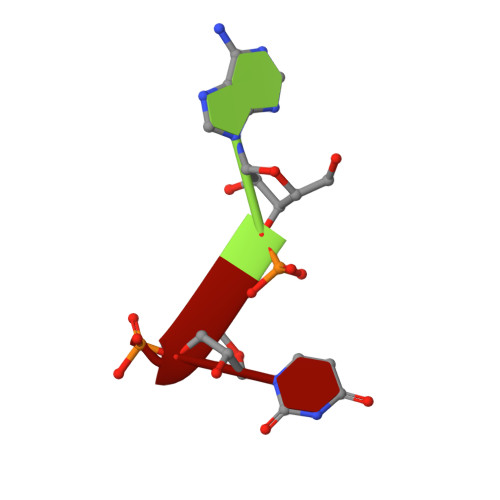
Characterization of SARS2 Nsp15 nuclease activity reveals it's mad about U.
Frazier, M.N., Dillard, L.B., Krahn, J.M., Perera, L., Williams, J.G., Wilson, I.M., Stewart, Z.D., Pillon, M.C., Deterding, L.J., Borgnia, M.J., Stanley, R.E.(2021) Nucleic Acids Res 49: 10136-10149
- PubMed: 34403466
- DOI: https://doi.org/10.1093/nar/gkab719
- Primary Citation of Related Structures:
7N06, 7N33 - PubMed Abstract:
Nsp15 is a uridine specific endoribonuclease that coronaviruses employ to cleave viral RNA and evade host immune defense systems. Previous structures of Nsp15 from across Coronaviridae revealed that Nsp15 assembles into a homo-hexamer and has a conserved active site similar to RNase A. Beyond a preference for cleaving RNA 3' of uridines, it is unknown if Nsp15 has any additional substrate preferences. Here, we used cryo-EM to capture structures of Nsp15 bound to RNA in pre- and post-cleavage states. The structures along with molecular dynamics and biochemical assays revealed critical residues involved in substrate specificity, nuclease activity, and oligomerization. Moreover, we determined how the sequence of the RNA substrate dictates cleavage and found that outside of polyU tracts, Nsp15 has a strong preference for purines 3' of the cleaved uridine. This work advances our understanding of how Nsp15 recognizes and processes viral RNA, and will aid in the development of new anti-viral therapeutics.
Organizational Affiliation:
Signal Transduction Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, 111 T. W. Alexander Drive, Research Triangle Park, NC 27709, USA.